Overview of Performance
Service delivery environment
During the reporting period of 2023/24 financial year, National Treasury had to reprioritise budget and related programmes owing to fiscal challenges faced by government in the financial year under review. Accounting Officers and Accounting authorities were advised on specific measures required to achieve much-needed savings thereby, implementing cost containment measures and budget cuts within government institutions including SAHPRA.
The collection of retention of license fees has been a challenge over the past few financial years. To overcome this challenge SAHPRA appointed a debt collecting agent to assist with collection of long outstanding balances. There were litigation cases brought against SAHPRA during the year under review. These cases were primarily about challenging SAHPRA’s Regulatory Mandate and powers of SAHPRA on taking decisions. The Authority has been fostering better relationships with law enforcement and other regulatory agencies such as the South African Veterinary Council, South African Pharmacy Council and HPCSA in order to address contraventions to the Medicines Act and other legislation. The local demands for increased cannabis related economic activity in the medicines space has maintained high demand for the Authority’ services. The Authority continues to have high demand for licensing and cannabis cultivation inspections, with the Inspectorate unit seeing more demand for inspections related to manufacturing of extracts and testing of cannabis related medicines. As the Regulatory Authority and its functions becomes more visible to the public, the reporting of non-compliances to the Medicines Act has seen a doubling of investigations over the last two years, that the Authority’s Regulatory Compliance unit is required to investigate within 30 working days, as per the Annual Performance Plan Target. High demand also exists for the review of shipments being imported, especially through the port at OR Tambo International Airport, which has resulted in an additional Border Medicines Control Technician being recruited at this port.
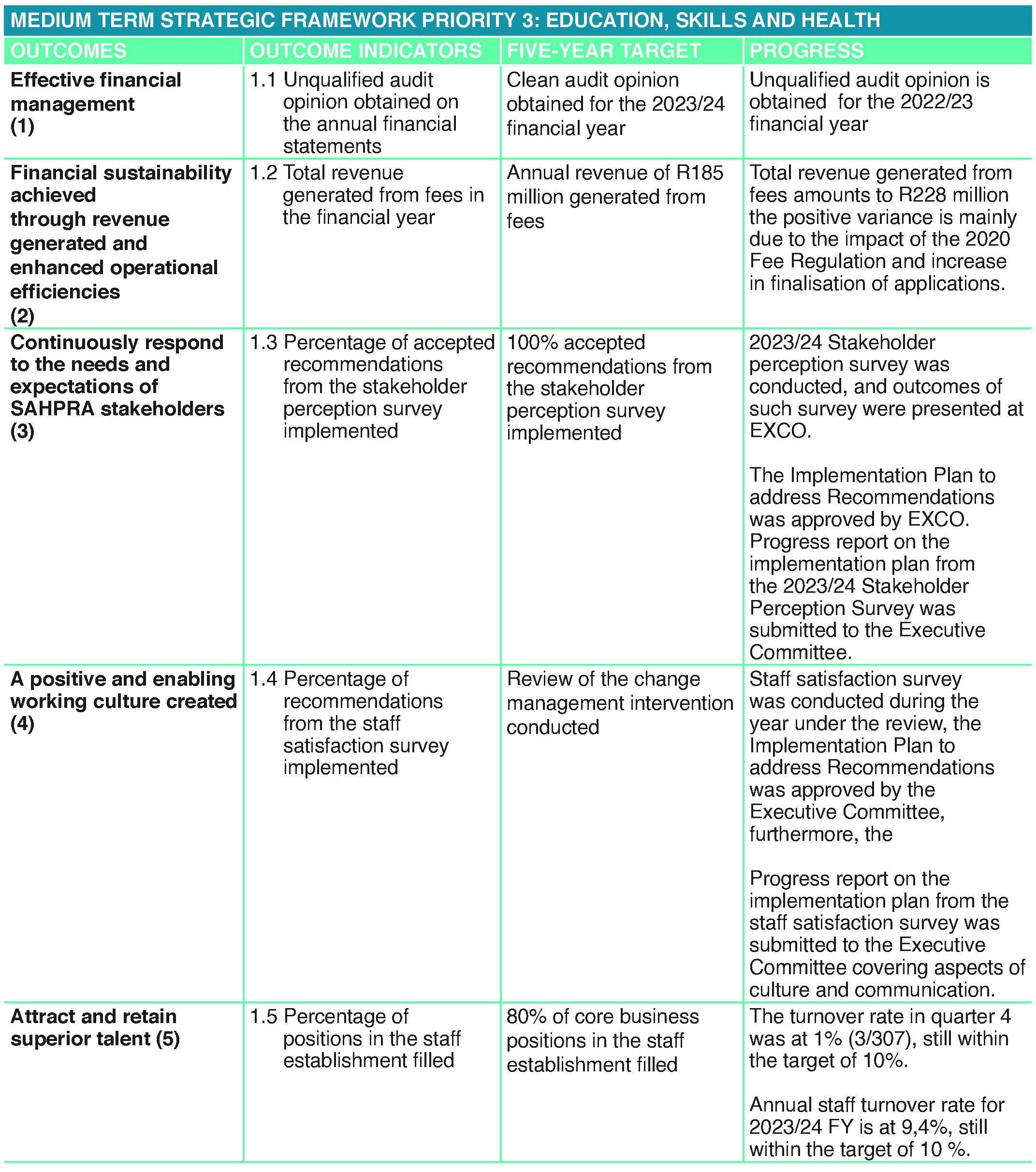
In terms of the performance against its 2023/24 Annual Performance Plan, the Authority planned to achieve 25 targets. Of the 25 planned targets, the Authority was able to achieve 17 targets which equates to 68% achievement, as depicted in the graphs on page 30:
During the period under review the South African Health Products Regulatory Authority was able to deliver on 17 of its 25 predetermined targets. Such key achievements can be summarised as follows:
- Current ratio of 1≥1 was maintained by achieving 1.16:1. In essence, SAHPRA’s total revenue amounted to R415.8 million against a budget of R381 million due to additional external funding support received during the year.
- 100 % New Chemical Entities were finalised within 400 working days.
- 86% generic medicines finalised within 250 working days.
- SAHPRA successfully managed to attain the International Organisation for Standardisation (ISO) 9001 Certification by SABS.
- 94% permits were finalised within 20 working days.
- 90% applications for the sale of unregistered Category A (human) medicines were finalised within 3 working days.
- 91% human clinical trial applications finalised within 80 working days.
- 126,6% medical device establishment license applications were finalised within 90 working days
- 116% applications for listed-electronic products finalised within 30 Working days.
The Authority did however experience varying levels of under-achievement against planned targets due under-capacitated business units, struggle to attract and retain the best talent especially in the technical field due to competition for best talent in the health, regulatory and pharmaceutical market. In terms of the targets that experienced challenges resulting in non-achievement, the Authority has identified the reasons for such deviation and has put in place relevant catch-up plans and mitigating measures so as to ensure success going forward.
Organisational environment
During the year under review, employees indicated several dissatisfaction matters that have led SAHPRA to commissioned Enterprises University of Pretoria to conduct an employee satisfaction survey. The survey was conducted from 18 – 28 September 2023. The aim of the survey was to gain an understanding of employees’ opinions and feelings about their workplace, their job and their work environment for SAHPRA to be able to address those areas where employees’ experiences are less than ideal. The report shows that staff is excited to work for SAHPRA. They have a strong sense of purpose and direction at SAHPRA, and they want to be part of SAHPRA’s future, believing that their work contributes to the overall success of SAHRA and the future vision of SAHPRA is important to them. Most employees have also indicated that they enjoy the hybrid working model as it creates flexibility and believes to improve staff morale, which shows that the entity has remote working capabilities than other organisations.
During the period 2023/24 FY, SAHPRA was under enormous constraints due to reduced funding from National Treasury, therefore, the organisation had to embark on strategies to cut operational costs and increase revenue collection, which included the reduction in advertisements of some of the funded positions. As a result, Business Units had to prioritise activities and utilise some of the external experts in achieving its mandate.
The implementation of the presence of SAHPRA technicians at the port has been effective in highlighting import non-compliances, thus being an effective mechanism for the detection and prevention of substandard, falsified or unauthorised product. To improve transparency in the processes for processing shipments, the Regulatory Compliance unit published workflows on the SAHPRA website to guide applicants and importers on requirements for importing health products subject to SAHPRA’s mandate. Improved processes and escalation procedures were implemented to improve service delivery and prevent delays at the ports. The Licensing Unit improved the regulation of the import of scheduled substances by completing the guideline for the Licence to Distribute Scheduled Substances and also successfully licensed two establishments. Controls in this framework includes the requirement to submit to SAHPRA for authorisation the lists of substances imported.
For the year under review, SAHPRA had the following targets to bolster its capacity:
- Conduct Employee Satisfaction Survey and implement the Action Plan to address recommendations from the survey report.
- Ensuring that 70% employees are trained to develop their skills they need to perform their duties, advance their careers, and keep abreast of continually changing business operations.
- Ensuring that 95% budgeted positions on the Recruitment Plan are filled to capacitate SAHPRA in achieving its mandate.
- Ensuring that the staff turnover rate is less than 10% or below to retain compete and experienced employees.
SAHPRA’s recruitment, selection and retention difficulties persisted. Forty-six (46) positions were funded to be filled for the 2023/24 financial year. However, only 67,4% (31/46) of those positions were filled as at 31 March 2024. Delays in filling of the positions were caused by completion of compulsory candidate pre-suitability checks (verification processes), re-advertising of positions because no suitable candidates were found during the selection processes or offers were declined by suitable candidates, just to mention few. This resulted in serious capacity constraints within SAHPRA and also placed additional strain on the remaining employees who had to carry the additional workloads.
SAHPRA has made considerable progress in ensuring that its employee profile is highly representative of the demographic profile of South Africa. In achieving Employment Equity (EE) targets, as set in the EE Plan, during the year (2023/24), 61% (19/31) of new appointments were women. The entity had the highest representation of Africans at 84% (259/309), followed by Indians at 6% (19/309), Whites at 5.5% (17/309) and the Coloureds with just 4.5% (14/309). The overall female representation is 62%, and 52,6% of females occupy senior and executive positions. Disability representativity is currently standing at 2%, maintained from the previous reporting year of 2022/23.
The staff turnover rate for the 2023/24 FY was at 9,4%, a decrease by 0,39% compared to last year. SAHPRA continued to experience a turnover on critical and scarce positions resulting in instability and a lack of continuity at management and operational levels. The turnover is attributable to terminations with reasons related to job security, career growth, work-life balance, remuneration and benefits, retirement and contract expiry were reasons why employees leave the services.
Delays in appointment of vacant position due to retirements and resignations. Below are challenges experienced by SAHPRA on Medical Device regulation. Delays in the finalisation of the second version of the Medical Device Regulations. The document initially was published for public comments in April of 2021 and republished for public comment in August of 2023 but still not yet finalised for implementation. Upon review of the latest comments received and engagement with the industry, further delays are anticipated as the industry are requesting a Regulatory Impact Assessment (RIA) report regarding the version 2 of the Medical Device Regulations. Manual processes for tracking submissions and approval statuses still persist. Manual tracking systems of large data can result in human error. At the end of 2023-24 FY, the tracking system used involved the manual recording of large amounts of data at key steps in the process for new medicine registrations on Google Sheet. The limited functionality on Google Sheets resulted in manual calculations for performance reporting as there is no stop clock mechanism available to perform the required calculations for timelines. The use of manual systems, especially for collection, maintenance of database, and follow-up on retention fees with the applicants, tends to be unreliable. Changes to the approval system (i.e., Signiflow) within the organisation affected the uploading and downloading of approved licences and permits on the system.
The Authority has attained its ISO 9001:2015 certification following a rigorous audit by the South African Bureau of Standards (SABS), a milestone that serves as a testament to the implementation of an effective and robust organisation-wide Quality Management System (QMS). A fully functioning QMS is core to achieving quality objectives that ensure that health products in South Africa meet statutory and regulatory standards of quality, safety, and efficacy. The continued improvement to the Quality Management Systems (QMS) that brought awareness required to ensure the control and use of current policies and documents, training on new and reviewed processes, internal collaboration with other units such as Regulatory Compliance, client-focused approach solutions, through engagement and transparency. Effective utilisation of resources. Implementation of the evaluator coordinator role in the new medicines workstream. The evaluator coordinator role played a significant role in closing the gap on the misalignment of allocations of technical screenings and evaluations in the different technical units. This resulted in overachievement in Q3 and Q4 and the overall success of the targets in the 2023-24 FY. Resignation of the HPA Senior Manager at the end of Q1 2023. However, the delegation of responsibilities was to a very experienced Manager in HPA. There was no resultant impact on the Annual Performance Plan for the unit.
With regards to capacity building and skills development, the Authority’s Inspectorate Unit participated in initial surveys on competency for a SAHPRA competency standard, where various inspectors participated covering a wide skillset. To enhance the issuing of permits and to improve the accuracy of reporting to the INCB, discussions were held with the UNODC/INCB regarding the procurement of the NDS7 tool, which will digitize the receipt, processing and issuing of permits. This will allow better control on reporting on narcotics and psychotropic. Due to ongoing discussions about the ability of the supplier of the tool to meet SAHPRA procurement process requirements, the procurement of the system was delayed. With the support of the South African Mission in Vienna in the fourth quarter, progress towards procuring this system was made and implementation is expected in the new financial year. The area is expected to become more effective with the accuracy of data capture required by the INCB for the control of narcotic and psychotropic substances. The use of online system will assist in ensuring that resources are allocated job specific function and ensure continuous improvement of the Authority’s business units. Various strategies are being utilised by the evaluation teams which include the implementation of different forms of reliance including participating in ZAZIBONA, which is a collaborative process, for the evaluation of new medicine applications and making use of assessment reports from Recognised Regulatory Authorities and SAHPRA.
Key policy developments and legislative changes
SAHPRA amended the Schedule 6 inscription for Tetrahydrocannabinol to make provision for a wider industrial application and use of the cannabis plant, which would then be subject to regulation under other government departments such as the Department of Health Food Directorate and the Department of Agriculture, Land Reform and Rural Development. These amendments were published for comment, and finalisation of the comments and publishing of the amended schedule inscription is expected in the new financial year.
As a result of an amendment to Regulation 3 of the Medicines Act, SAHPRA was required to develop and publish a draft guideline for medicine compounding for comment. The comments were received and are currently under review. The publishing of a finalised compounding guideline will add further regulation to the area of compounding.
Progress towards achievement of institutional Impacts and Outcomes
SAHPRA’s revised 2020/21 – 2024/25 Strategic Plan was approved in January 2023. The revisions were made to the to ensure alignment with revisions in the Annual Performance Plan based on the recommendations from the audit by Internal Audit, Outcome indicators, 5-year targets and method of calculation in the Technical Indicator Descriptions.
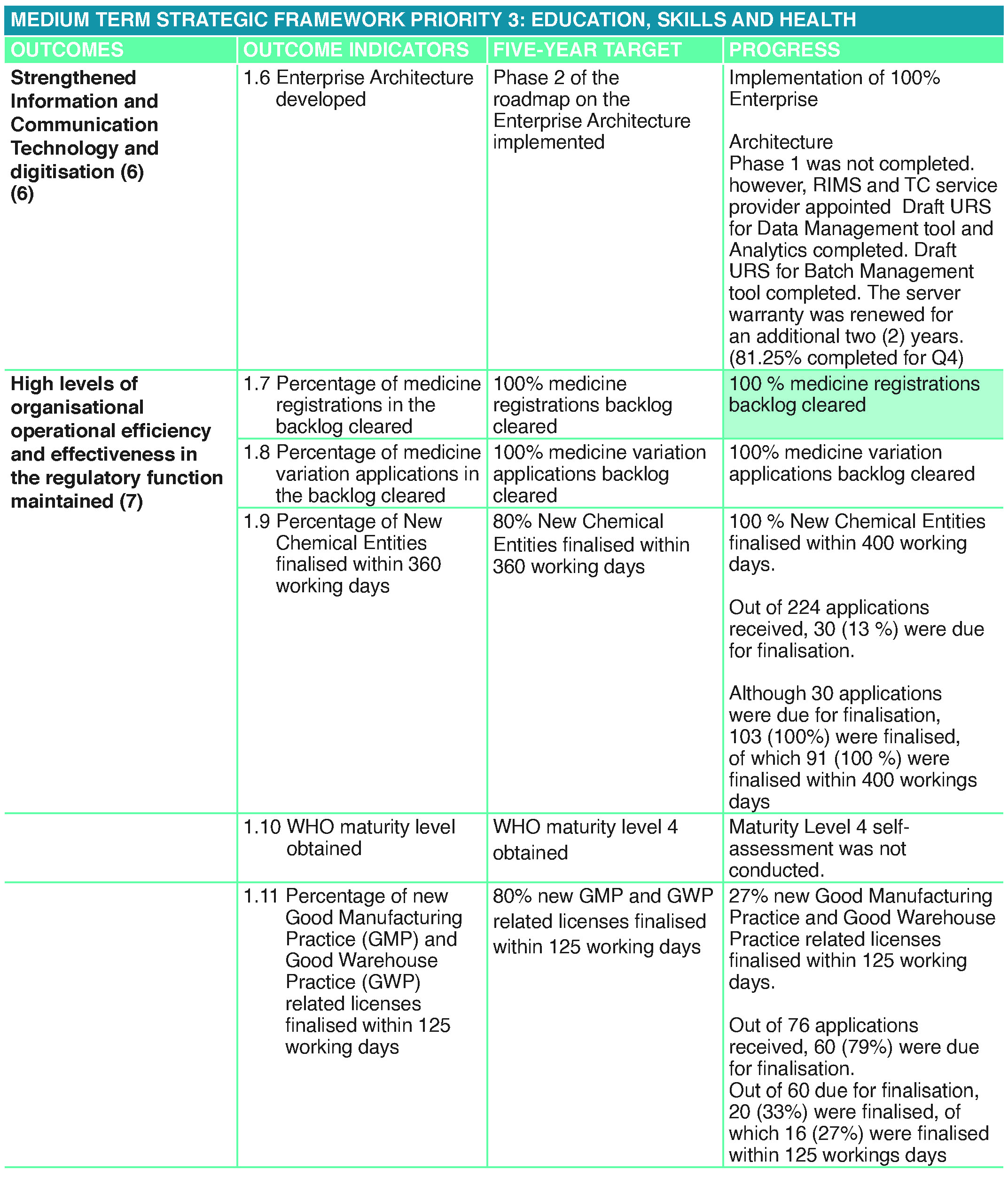
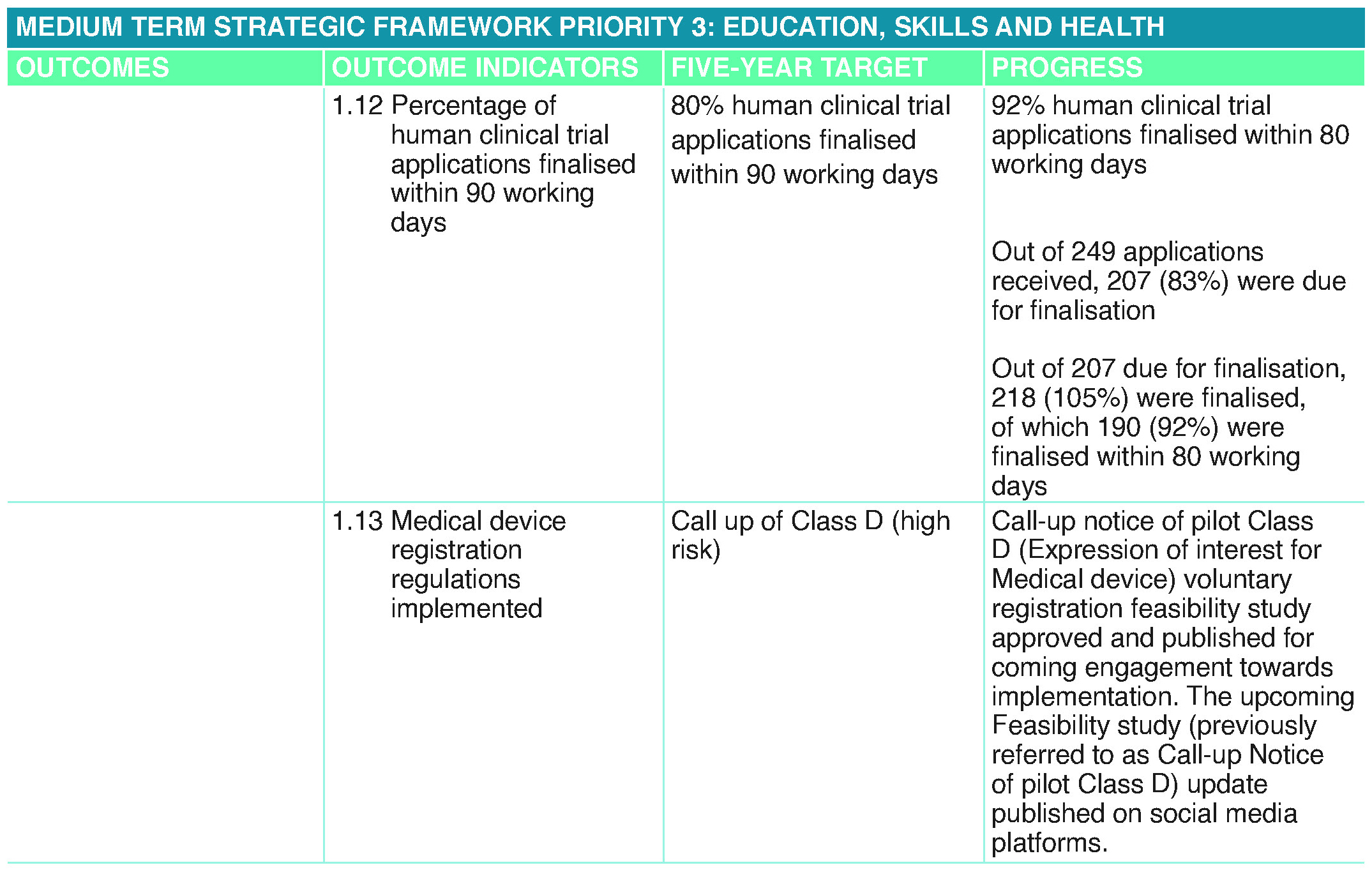
Progress on Outcomes