Institutional Programme Performance Information
Programme 1: Leadership and Support
Purpose: To provide the leadership and administrative support necessary for SAHPRA to deliver on its mandate and comply with all legislative requirements.
Sub-programmes

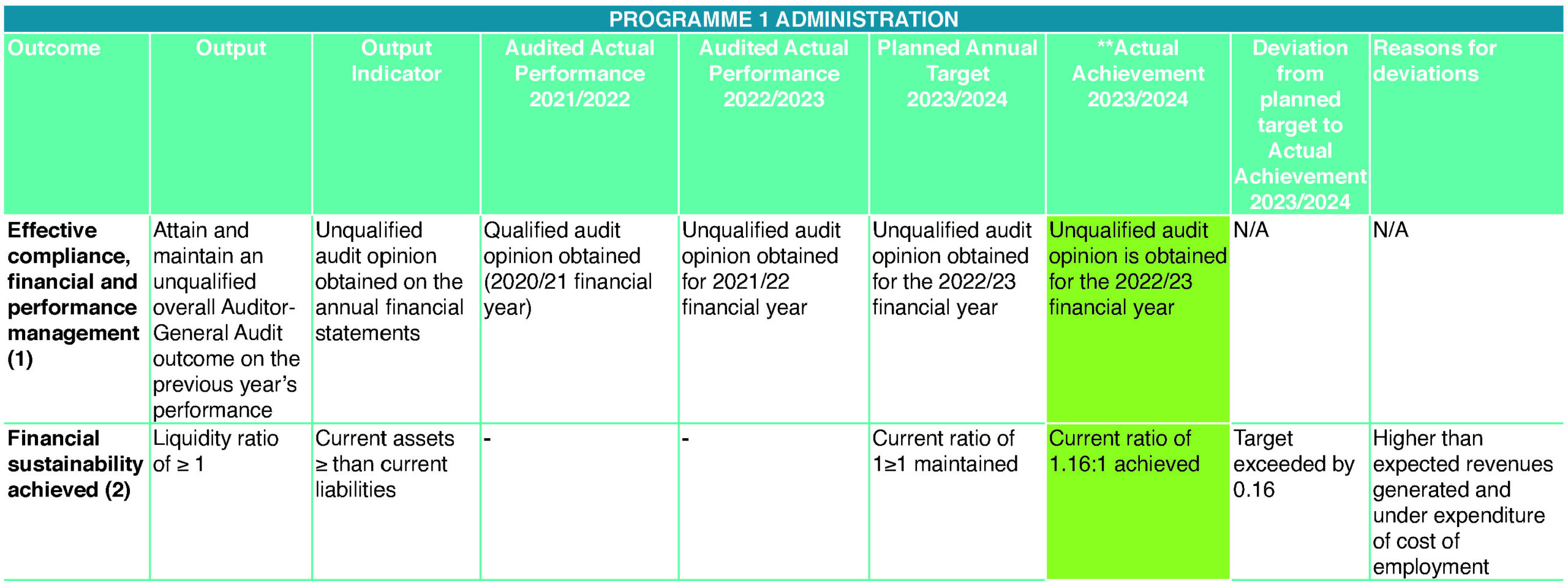
Effective compliance, financial and performance management (1)
- Financial sustainability achieved (2)
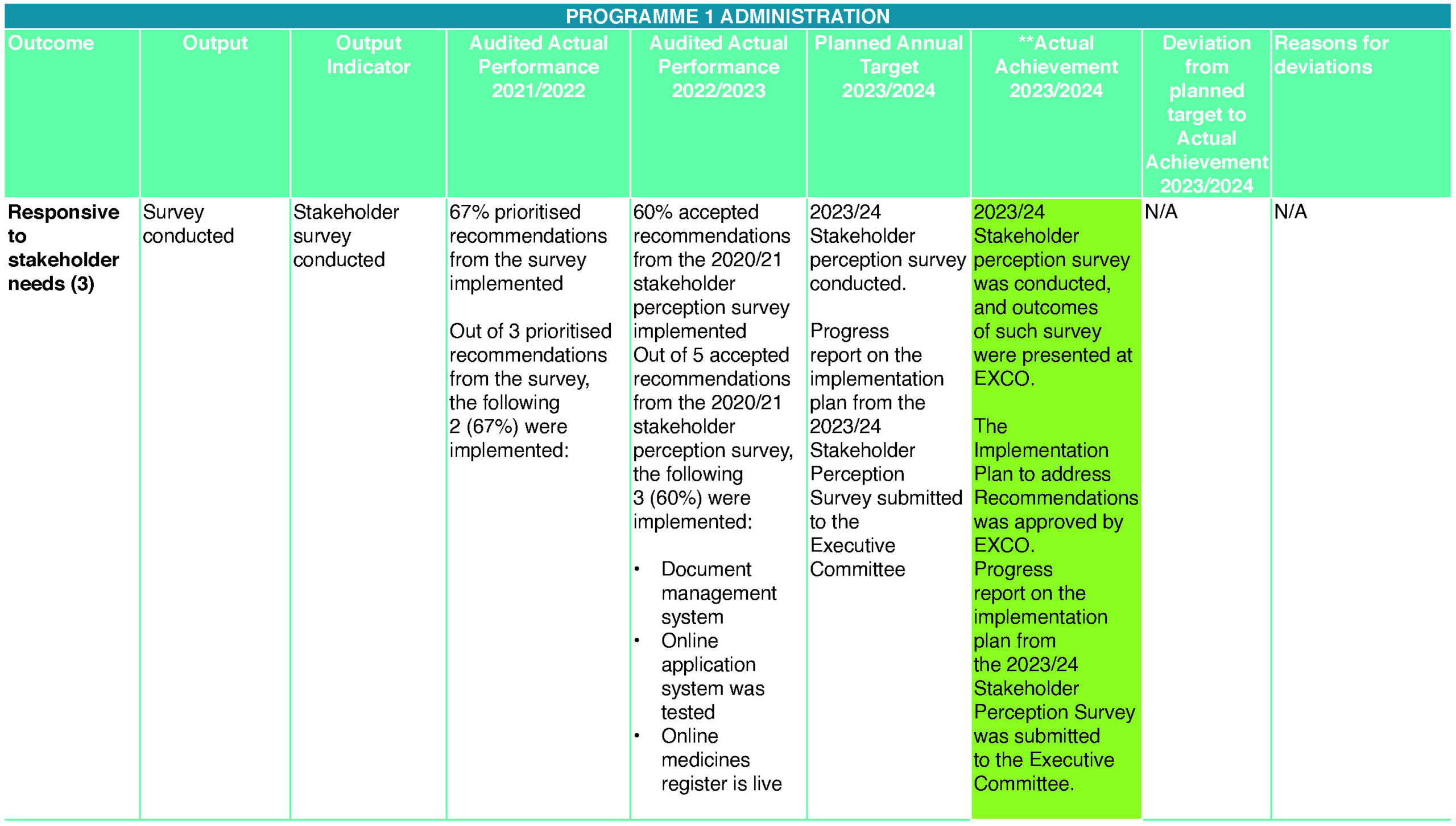
- Responsive to stakeholder needs (3)
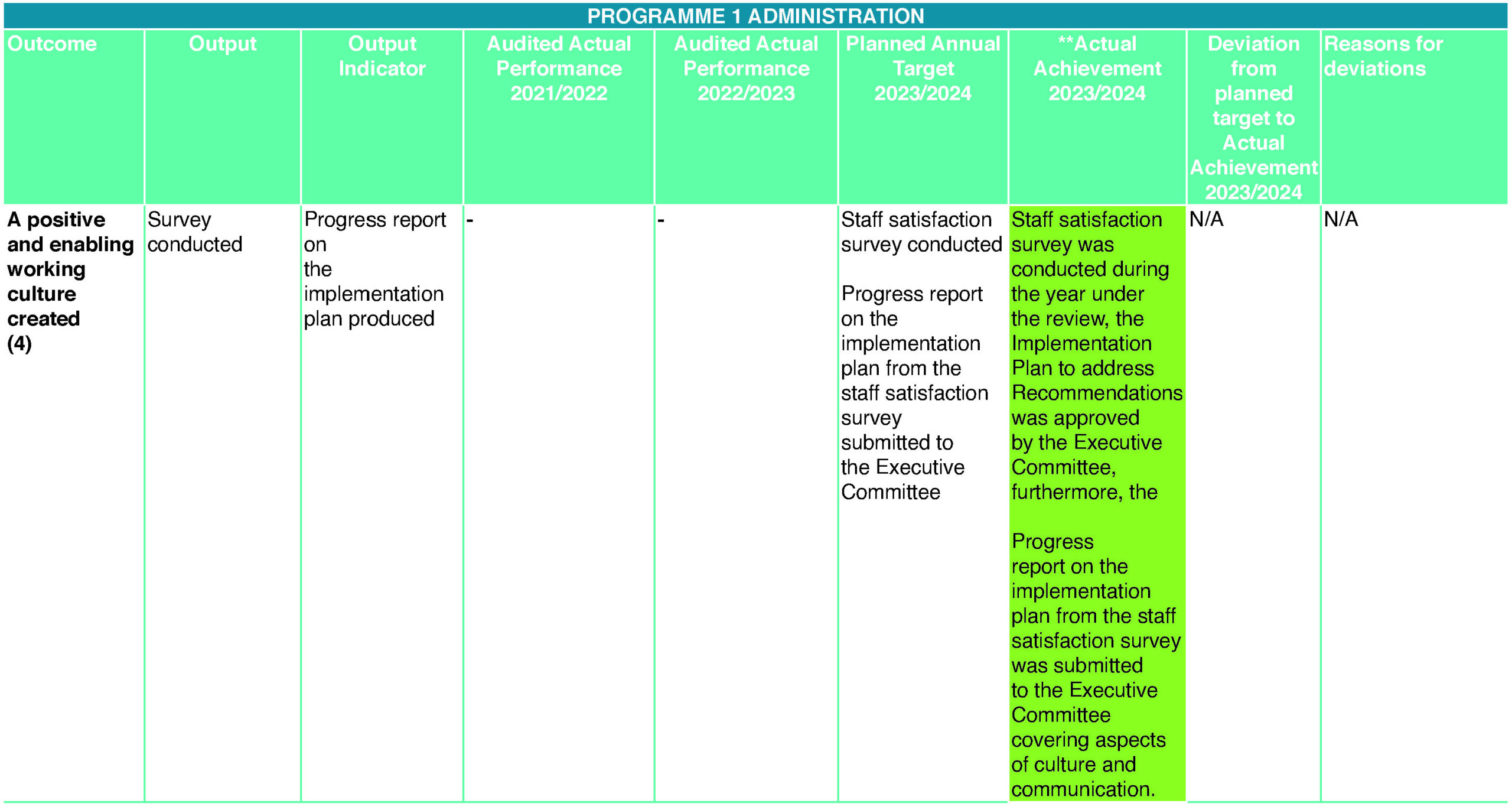
- A positive and enabling working culture created (4)
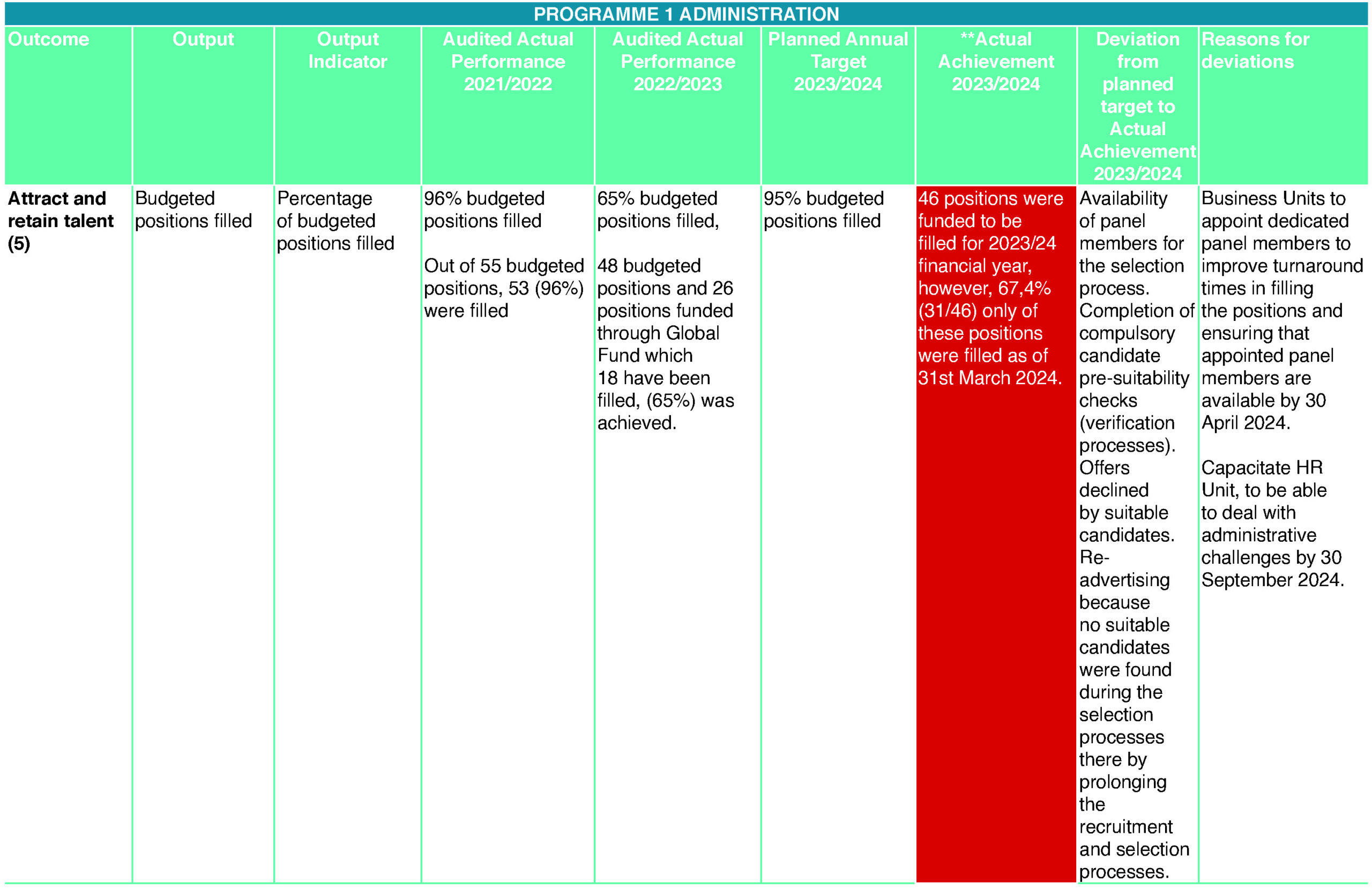
- Attract and retain talent (5)
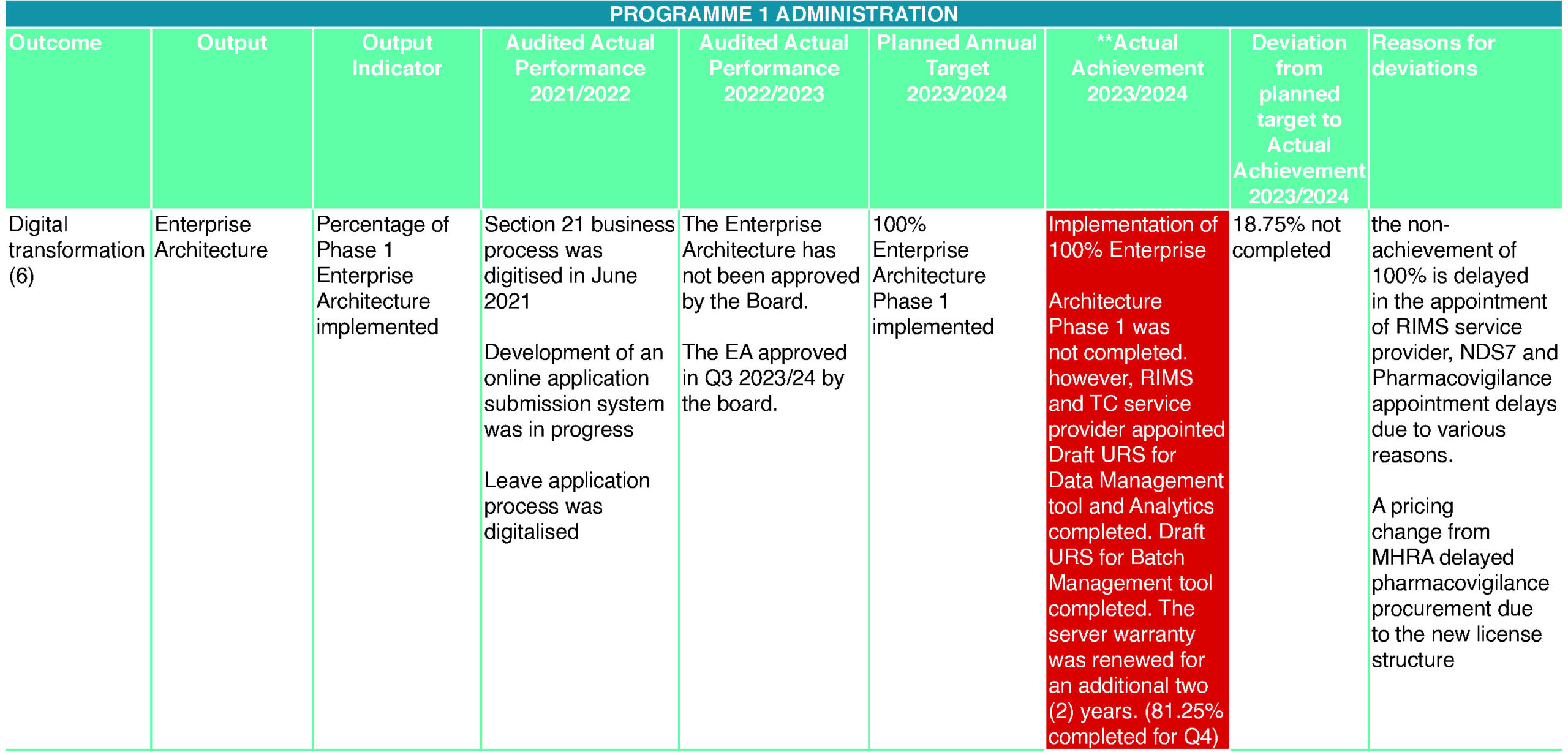
- Digital transformation (6)
Outcomes, Outputs, Output Indicators, Targets and Actual Achievements
Financial and Supply Chain Management
SAHPRA’s total revenue amounted to R435.4 million against a budget of R381 million. The variance of R54.5 million was mainly due to additional external funding support received during the year and additional fee revenue derived. SAHPRA spent R424 million against the initial approved budget of R381 million. The additional expenditure was allowed due to unbudgeted for external financial support received as well as above-budgeted for fee income generated during the year. The overall result was an accounting surplus amounting to R11 million and exceeding the annual cash flow ratio target of 1:1.
The focus was on improving previous audit outcomes and positioning SAHPRA for financial sustainability. The entity has:
- Achieved an unqualified audit (2022/23)
- Enforced finance and supply chain policies and standards, reducing irregular, fruitless and wasteful expenditure.
- Revised service fee structures submitted for approval and gazetting.
Communication and Public Relations
The annual achievement for the unit is the implementation of the communication strategy through tailored and targeted messaging via communication channels (website, emails, webinars/workshops, social media channels) across different audiences (public/industry/healthcare professionals/partner organisations, etc.) to enhance and educate stakeholders on key public issues around the safety of health products in the country.
The unit’s communication campaigns (around awareness of the dangers of substandard/falsified medications to reporting side effects to name a few) directed to external stakeholders (public/industry/healthcare professionals/partner organisations etc.) continue to bring awareness of SAHPRA’s mandate of protecting the health and well-being of South Africans, with a direct impact on one of government’s strategic priorities of education. SAHPRA’s messaging is about informing and educating stakeholders about the Regulator’s work to protect the public’s health and well-being. Lack of budget to support the unit’s needs in terms of collateral production like pamphlets, manuals, guides, etc, led to the unit being unable to deliver better access to information for key stakeholders such as public and healthcare professionals. The retirement of the Manager: Communication and PR created challenges for the Acting team member to address all key deliverables and their targets.
Human Resource Management
During the year under review, employees indicated several areas of dissatisfaction that led SAHPRA to commission an external service provider, Enterprises University of Pretoria, to conduct an employee satisfaction survey. The survey was conducted from 18 – 28 September 2023. The survey aimed to gain an understanding of employees’ opinions and feelings about their workplace, their job and their work environment for the organisation to be able to address those areas where employees’ experiences are less than ideal. The report shows that the staff is excited to work for SAHPRA. They have a strong sense of purpose and direction at SAHPRA and want to be part of SAHPRA’s future, believing that their work contributes to the overall success of SAHPRA and the future vision of the entity is essential to them. Most employees have also indicated that they enjoy the hybrid working model as it creates flexibility and believe it contributes to improving staff morale, which shows that the entity has remote working capabilities.
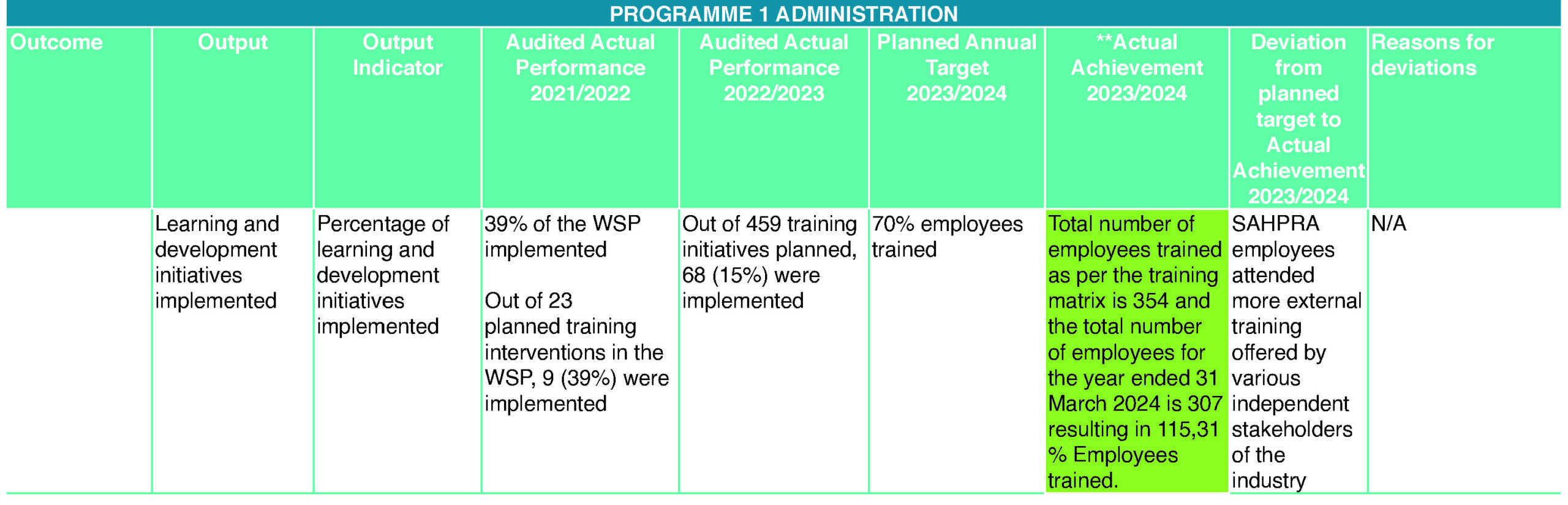
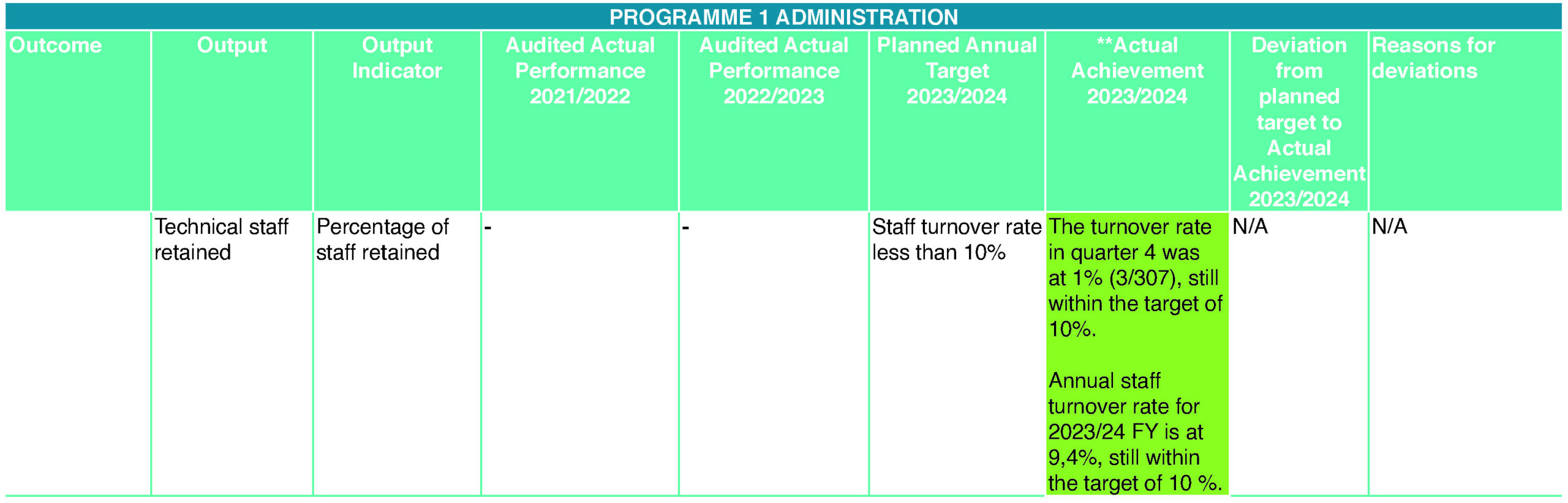
SAHPRA provided bursaries and training opportunities to employees to further their qualifications and develop the skills they need to perform their duties, advance their careers and keep abreast of continually changing business operations. For 2023/24, the target was to train 70% (215) employees. A total of 351 (114,7%) employees were trained from April 2023 to March 2024. Most of the training was offered by various independent external industry stakeholders. The entity has made considerable progress in ensuring that its employee profile is highly representative of South Africa’s demographic profile. The entity’s composition is 62% women, 21% youth (28 – 35 years) and 2% people with disability. Women’s representation in senior and executive management (SL 13 – 16) is at 52,6%. The entity has achieved the national target of 50% women at senior management level and 2% of people living with disabilities as set by Parliament. In the new financial year, the entity will enter partnerships with NDOH and continue to support youth employment initiatives through the implementation of the Community Service Programme for graduates in a bid to address youth unemployment thus providing them with an opportunity to gain relevant experience.
Information and Communication Technology (ICT)
The ICT service delivery for SAHPRA is aligned with the Corporate Governance ICT Framework (CGICT) and is aimed at enabling the Authority to produce measurable results toward achieving its ICT strategies and goals. The implementation of the Regulatory Information Management System (RIMS) together with the technology consultancy projects are aimed at enhancing the Authority’s service delivery to the industry. The Authority faced challenges and delays in sourcing the correct service provider for the RIMS project, but eventually awarded it in the last quarter of the financial year 2023/24. RIMS and a technology consultancy service provider were appointed to implement the digital strategy and significant progress has been made in the digitisation project directly related to the Health Products Authorisation (HPA), Licensing, and Section 21 units.
The delivery of online digitised services relies heavily on the availability of internet services via internet Service providers. National and global internet disruptions can create delays in the delivery of services. The Authority’s ICT staff has set up redundancies to ensure business continuity. Limited resources remain a challenge within the ICT Unit in the delivery of services. However, a total number of 4 527 calls were received through our Service Desk on all categories; 6% of total calls breached the SLA, and 94% of the total calls were resolved within the SLA for the financial year 2023/24. This is achieved by ensuring that incidents are closed only after providing a proper resolution and confirming with the end user, and applying the appropriate closure notes on the knowledge database. The ICT Architecture Framework is being used to translate SAHPRA’s Digitisation business strategy through the Single Cloud Framework. The SAHPRA environment maintained a security patch compliance rate of more than 95% during the fiscal year 2023/24.
Linking performance with budgets:

Strategy to overcome areas of under performance
Across the entity, recruitment, selection and retention difficulties persisted during the year under review. Forty-six (46) positions were funded to be filled for the 2023/24 financial year. However, only 67,4% (31/46) of those positions were filled as at 31 March 2024. Delays in filling of the positions were caused by the completion of compulsory candidate pre-suitability checks (verification processes), re-advertising of positions because no suitable candidates were found during the selection processes and some offers were declined by suitable candidates and budget cuts implemented by National Treasury, some positions were unfunded to remain within the allocated Cost of Employment (CoE) budget. This resulted in severe capacity constraints within the entity and placed additional strain on the remaining employees carrying the additional workloads. In correcting the situation, business units will appoint dedicated panel members to improve turnaround times in filling the positions and ensuring that appointed panel members are available. The HR Unit will be capacitated to deal with administrative and technical challenges. Digitisation of business processes, particularly the automation of SCM and claims processes through the appointment of service providers to develop such systems, continued to be applied to overcome under-performance in certain areas. The negative expenditure variance is mainly due to external funding received which was not budgeted for and the retention of prior year surpluses.
Programme 2: Health Products Authorisation
Purpose: To provide administration support necessary for SAHPRA to deliver on its mandate and comply with the relevant legislative requirements. The specific purpose of this programme is to coordinate the registration and/or licensing or amendment of applications in respect of medicines within a legislative framework. This framework defines the requirements for application to the Authority and for receiving, recording, and distributing all documents submitted to SAHPRA.
Sub-programmes

Outcomes
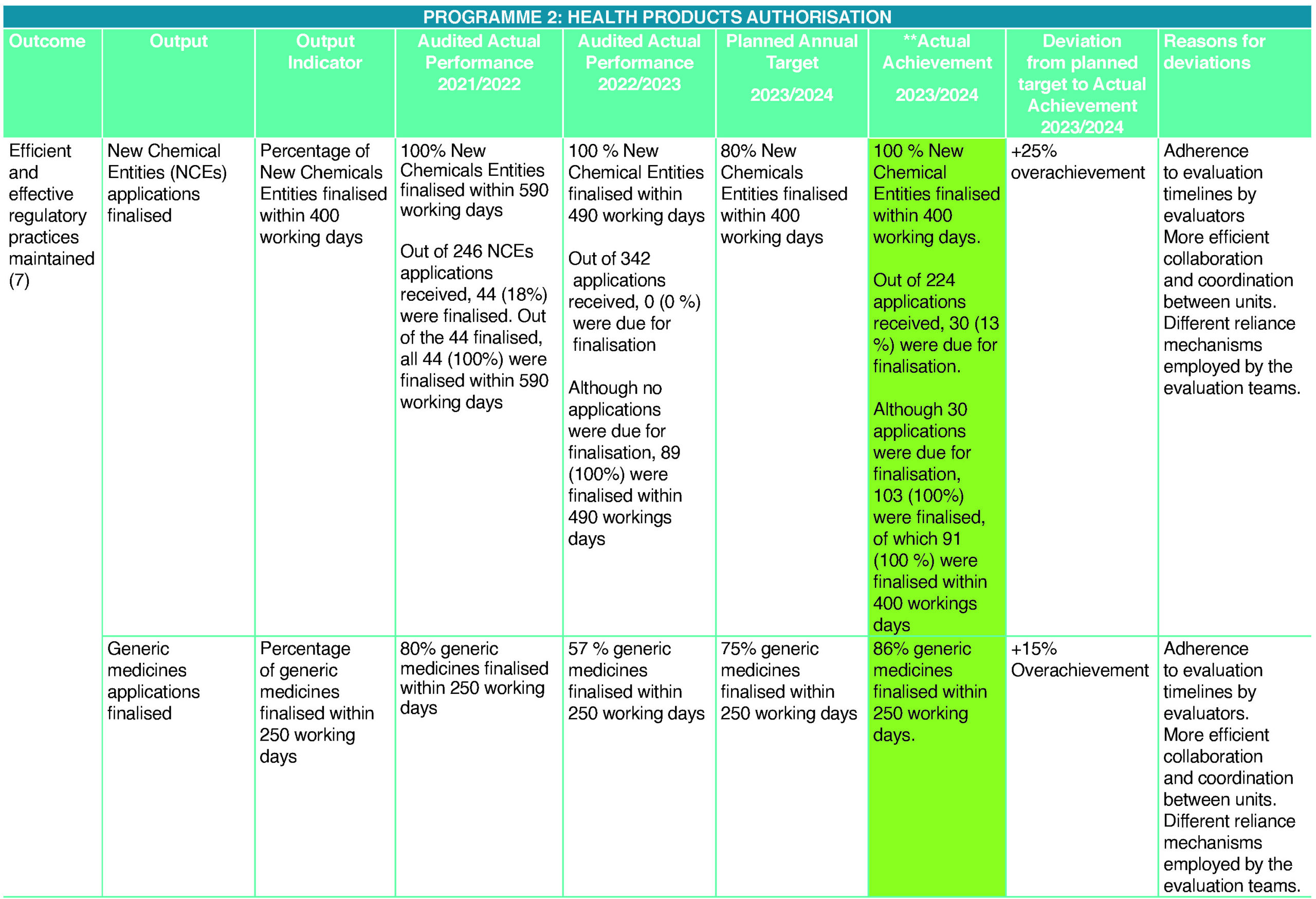
Efficient and effective regulatory practices maintained (7)
Global best practices maintained (8)
Outcomes, Outputs, Output Indicators, Targets and Actual Achievements
Health Products Authorisation (HPA)
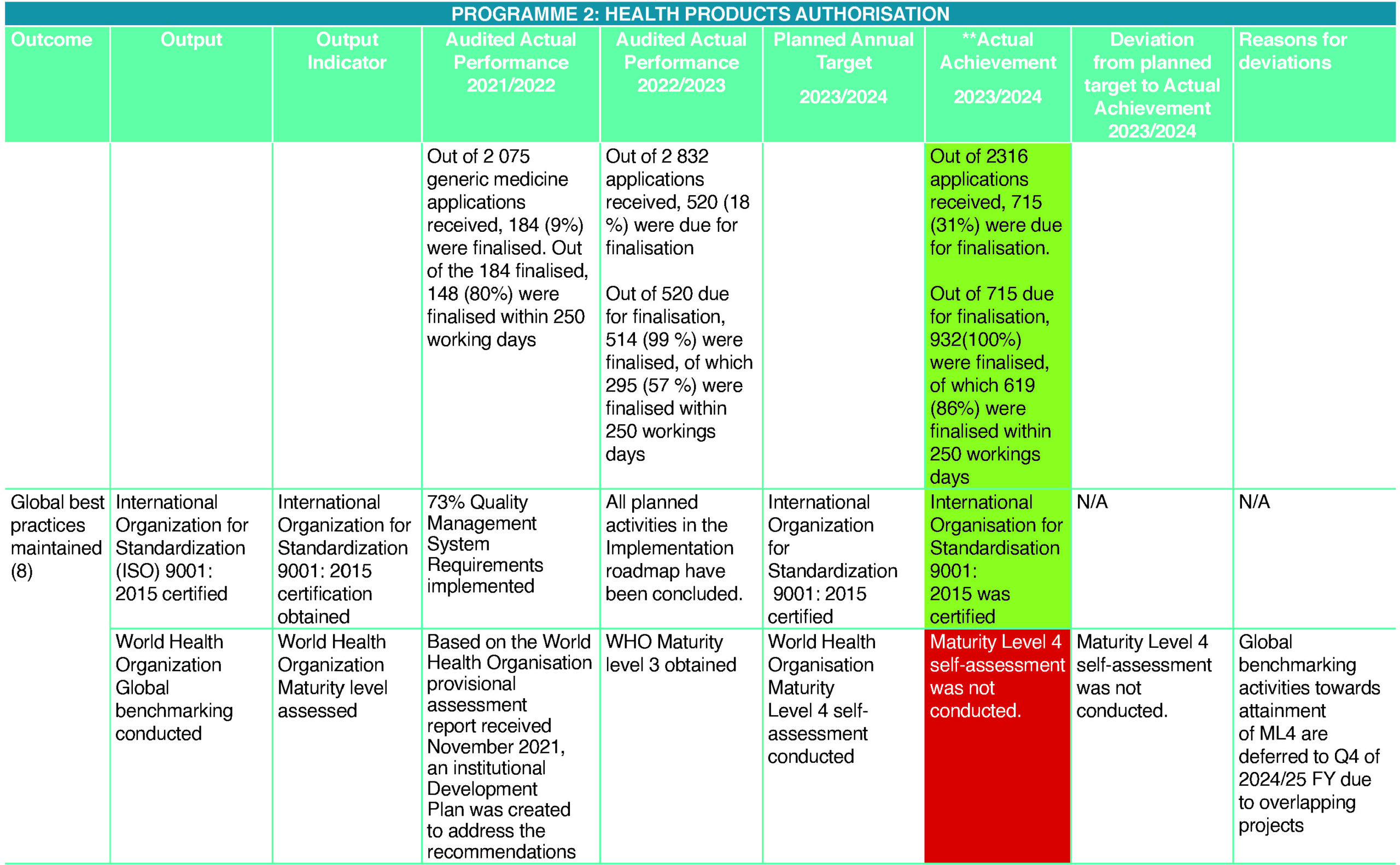
Out of 224 (220 human plus four veterinary) New Chemical Entity (NCE) applications received (inclusive of applications carried over from the previous Quarter), 103 (102 human plus one veterinary) (46%) NCEs were finalised. Thirty (26 human plus four veterinary) (13%) NCE applications were due for finalisation in the fourth quarter (Q4), 91 (100%) were finalised within 400 working days from the date of completion of technical screening. Out of 2 315 (2 277 human plus 38 veterinary) generic applications received (inclusive of applications carried over from the previous quarter), 715 (707 human plus eight veterinary) (31%) were due for registration by the end of the fourth quarter (Q4). Out of the 715 due for registration, 932 (100 %) were finalised, of which 619 (86%) were finalised within 250 working days from the date of completion of technical screening. The rest of the applications were finalised between 251 and 547 working days.
The evaluation teams are utilising various strategies, including implementing different forms of reliance, participating in ZAZIBONA, a collaborative process, for the evaluation of new medicine applications, and using assessment reports from RRAs and SAHPRA.
The registered products encompass therapeutic areas such as serum cholesterol reducers, antihypertensives, antihistamines, anticonvulsants, antidepressants, anticoagulants, anti-acids, anti-infectives, oncology, oral hypoglycaemics, sedatives, vascular medicines, ophthalmic preparations, immunosuppressants, contraceptive preparations, migraine preparations, corticosteroids, anti-parkinsonism preparations, and antiviral drugs.
HPA had some challenges during the year under the review. Manual processes for tracking submissions and approval statuses. Such kind of tracking systems of large data usually resulted in human error. In a nutshell, the tracking system used by HPA involved the manual recording of large amounts of data at critical steps of the process for new medicine registrations on Google Sheets. The limited functionality on Google Sheets resulted in manual calculations for performance reporting as there is no stopping clock mechanism available to perform the required calculations for timelines. Furthermore, the resignation of the HPA Senior Manager at the end of Q1 2023 left huge responsibilities. However, the new Senior Manager was appointed at the end of Quarter 4. SAHPRA continues its digital transformation journey by implementing RIMS with enhanced tracking and reporting capabilities. Data automation will result in faster and more accurate information handling and report generation.
Quality Management System (QMS)
During the year under review, SAHPRA underwent a voluntary independent certification audit conducted by the SABS to test the implementation of SAHPRA’s Quality Management System against the International Organisation for Standardisation (ISO) 9001 standard, a globally recognised standard for quality management systems developed by the International Organisation for Standardisation (ISO).
SAHPRA successfully attained the ISO 9001 certification by SABS. This milestone serves as a testament to the implementation of an effective and robust organisation-wide quality management system. This international certification confirms SAHPRA’s position among global peers as a provider of globally acceptable regulatory services that comply with or exceed the ISO 9001 international standard on quality management. The Authority continues its digital transformation journey with the implementation of RIMS with enhanced tracking and reporting capabilities.

Linking performance with budgets:

Strategy to overcome areas of underperformance
Various strategies are being utilised by the evaluation teams, which include the implementation of different forms of reliance, including participating in ZAZIBONA, which is a collaborative process, for the evaluation of new medicine applications and making use of assessment reports from RRAs and SAHPRA. Furthermore, capacitating the organisation with the right skills is significant in achieving the objectives of the Authority. The implementation of RIMS will continue together with the technology consultancy projects; in a nutshell, the digitation of the processes remains the priority of the organisation to ensure operational efficiencies.
Programme 3: Inspectorate and Regulatory Compliance
Purpose: To ensure public access to safe health products (including disclaimers) through inspections and regulatory compliance. The focus of this programme is on the assessment of site compliance with good regulatory and vigilance practices, including:
- Good Manufacturing Practice (GMP)
- Good Clinical Practice (GCP)
- Good Warehouse Practice (GWP)
- Good Distribution Practice (GDP)
- Good Laboratory Practice (GLP)
- Good Vigilance Practice (GVP)
Sub-programmes:

Outcomes
- Efficient and effective regulatory practices maintained (7)
Outcomes, Outputs, Output Indicators, Targets and Actual Achievements
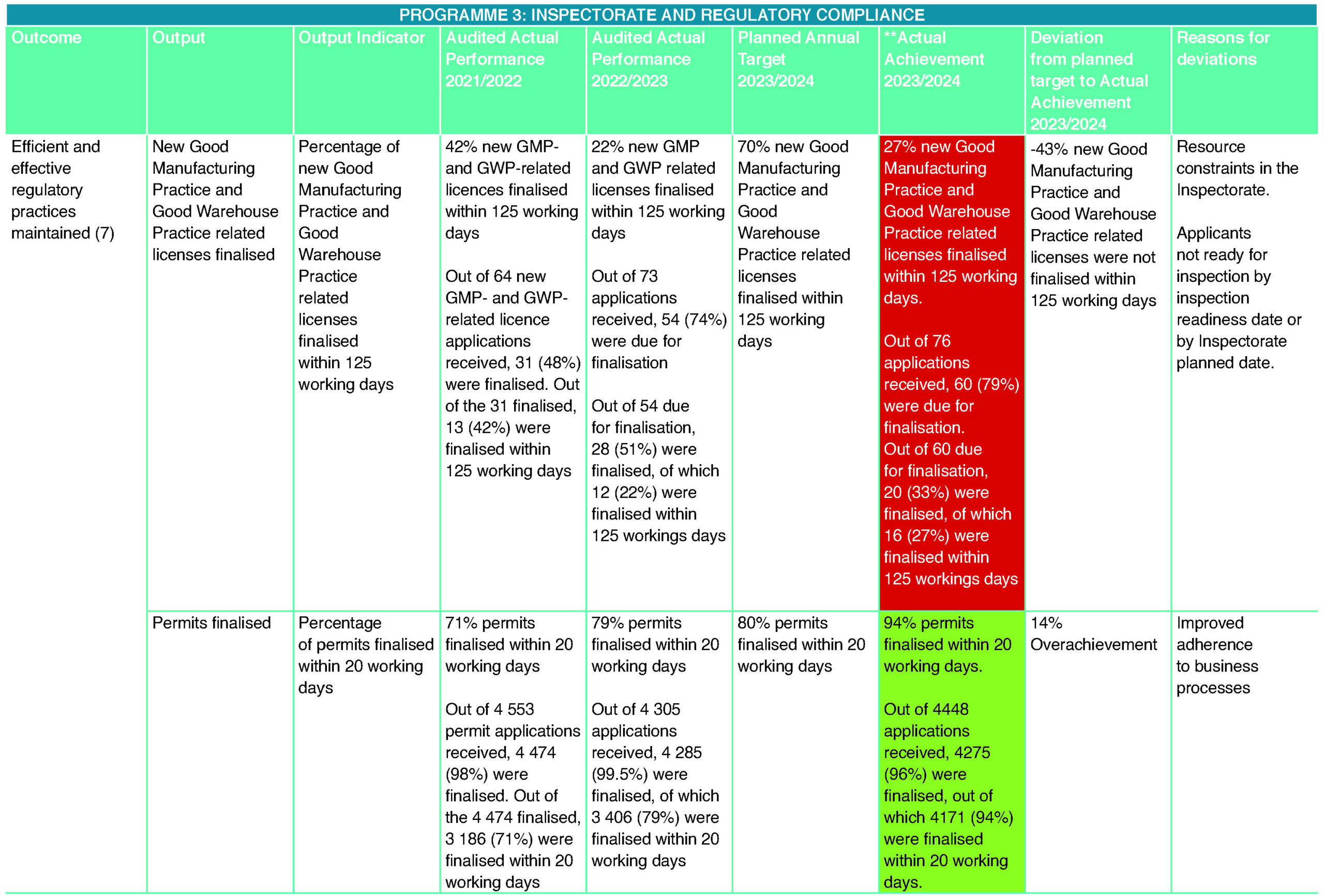
Of the three targets for Inspectorate and Regulatory Compliance, the programme consistently achieved the target for issuing permits within 20 working days. This contributed to the successful importation of narcotics and psychotropics needed for public health requirements as per the National Drug Policy, as well as contributing to the control of import and export of these controlled substances in line with South Africa’s requirement to adhere to INCB’s requirements.
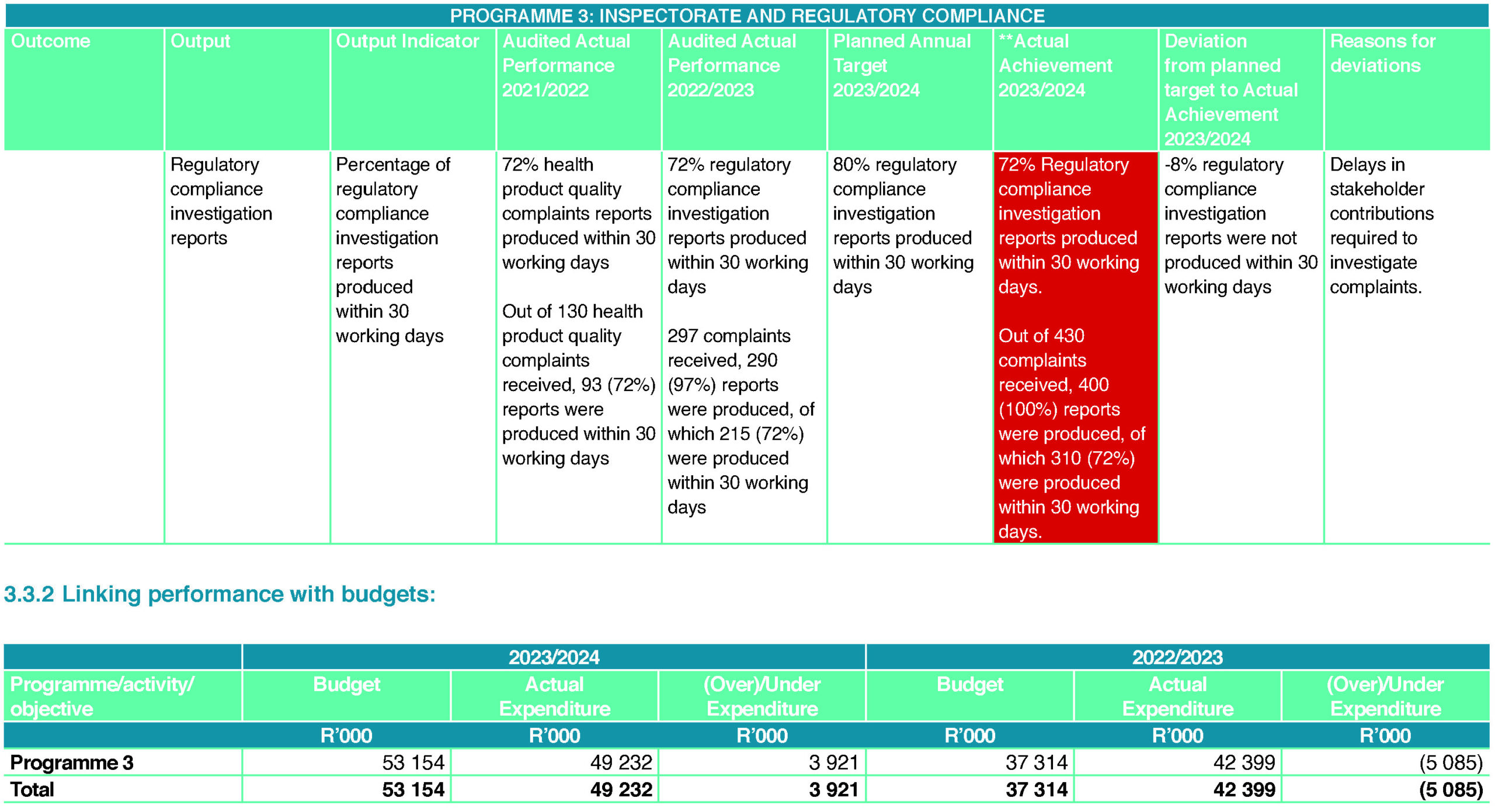
Due to the significant increase in reporting of the Medicines’ Act non-compliances, the resources assigned to the Regulatory Compliance Unit for investigations could not achieve the target set. This was also affected by the delay in input by stakeholders that SAHPRA collaborates with to investigate and enforce compliance through legislation. Due to a further expected increase in reported non-compliances, the target will be revised to align with resources and process requirements for the new financial year.
The target for issuing GMP- and GWP-related licences within 125 working days was not achieved due to the Inspectorate Unit’s capacity constraints and delays caused by applicant inspection readiness delays. For the coming financial year, the calculation of this target will not include time spent with the applicant where the applicant has delayed inspections due to the site not being ready for inspection.
Regulatory Compliance
The Regulatory Compliance Unit consistently met its target for the issuing of permits in this financial year. To enhance the business process for issuing permits and to improve the accuracy of reporting to the INCB, discussions were held with the UNODC/INCB regarding the procurement of the NDS7 tool, which will digitise the receipt, processing and issuing of permits. This will allow better control of reporting on narcotics and psychotropics. Due to ongoing discussions about the ability of the supplier of the tool to meet SAHPRA’s procurement process requirements, the system’s procurement was delayed.
Inspectorate
The Inspectorate Unit focused on training and capacitating newly recruited inspectors in the GMP area. Together with the Regulatory Compliance Unit, the GMP Inspectorate’s sub-unit underwent a risk-based GMP inspection training, provided by the WHO Regulatory System Strengthening team, which focused on sterile and biological manufacturing. Regarding capacity building and skills development, the Inspectorate Unit participated in initial surveys on competency for a SAHPRA competency standard, where various inspectors participated covering a broad skill set. The unit also focused on recruitment of GMP inspectors, which is expected to continue into the new financial year.
Licensing
The Licensing Unit performed well in meeting its target for licence renewals and amendments. However, the target for new licence applications was not achieved due to capacity challenges in the Inspectorate Unit and applicants’ inspection readiness. The inspection rate of new licence applications was insufficient to accomplish the APP target for new GMP and GWP licences. In the coming financial year, the time delayed as a result of the applicant’s readiness for inspection will be removed from the performance calculation.
Strategy to overcome areas of underperformance
To improve the operations at ports of entry, measures have been put in place and the appointment of personnel, to be stationed at major ports of entry, has been prioritised. To ensure improved coordination at ports of entry, SAHPRA has been engaging with relevant stakeholders such as the South African Revenue Services (SARS) and the NDoH (Port Health). Furthermore, capacitating the organisation with the right skills is significant in achieving the objectives of the Authority. The implementation of the RIMS will continue together with the technology consultancy projects; in a nutshell, the digitisation of the processes remains the priority of the organisation to ensure operational efficiencies.
Programme 4: Clinical and Pharmaceutical Evaluation
Purpose: To evaluate the safety, quality and therapeutic efficacy of medicines and register them for use as per the delegated authority and in terms of the relevant legislation, as listed in the legal mandate in part 1a of the strategic plan.
Sub-programmes

Outcomes
Efficient and effective regulatory practices maintained (7)
Outcomes, Outputs, Output Indicators, Targets and Actual Achievements
Sale of Unregistered Category A (Human) Medicines
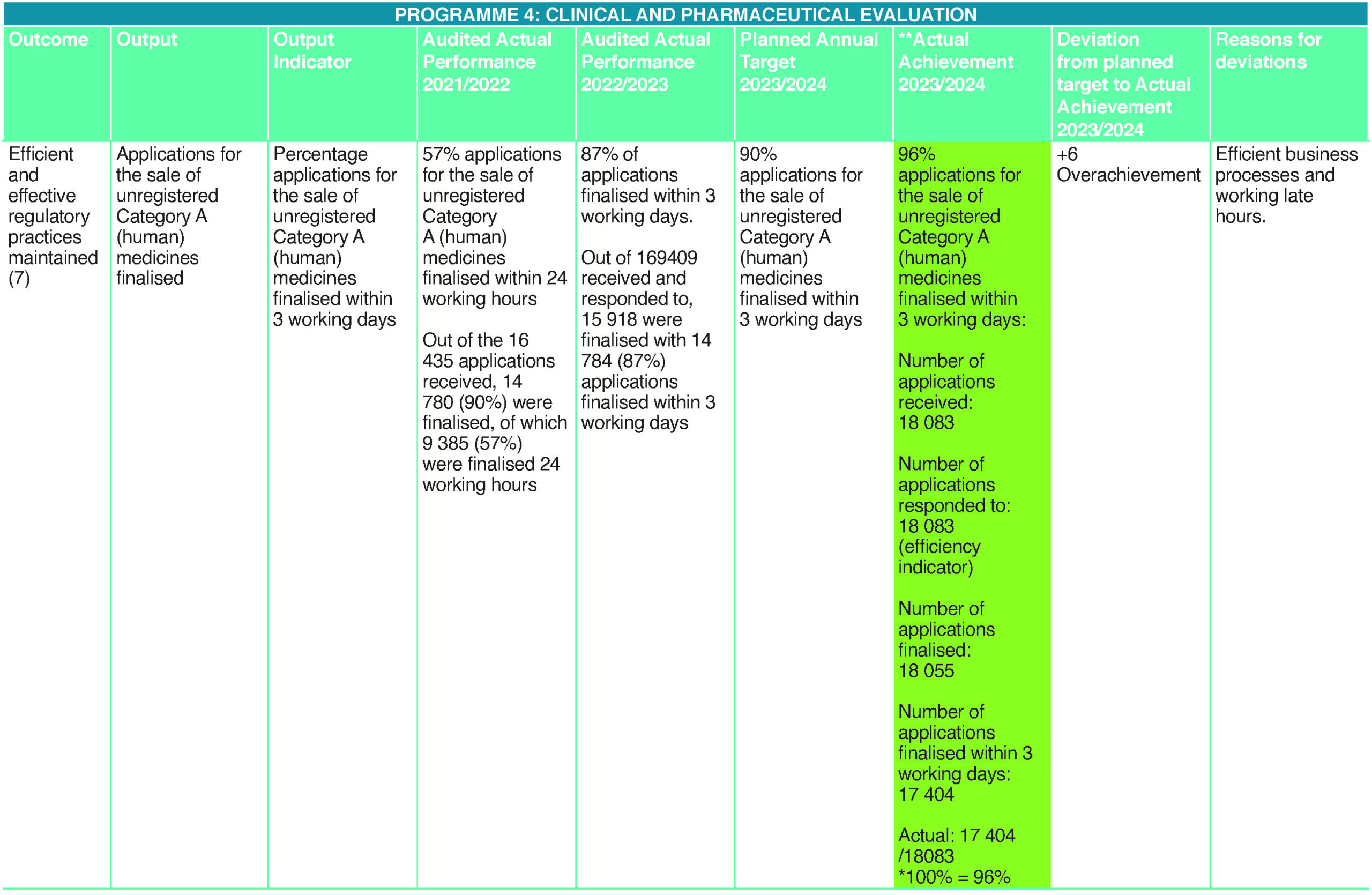
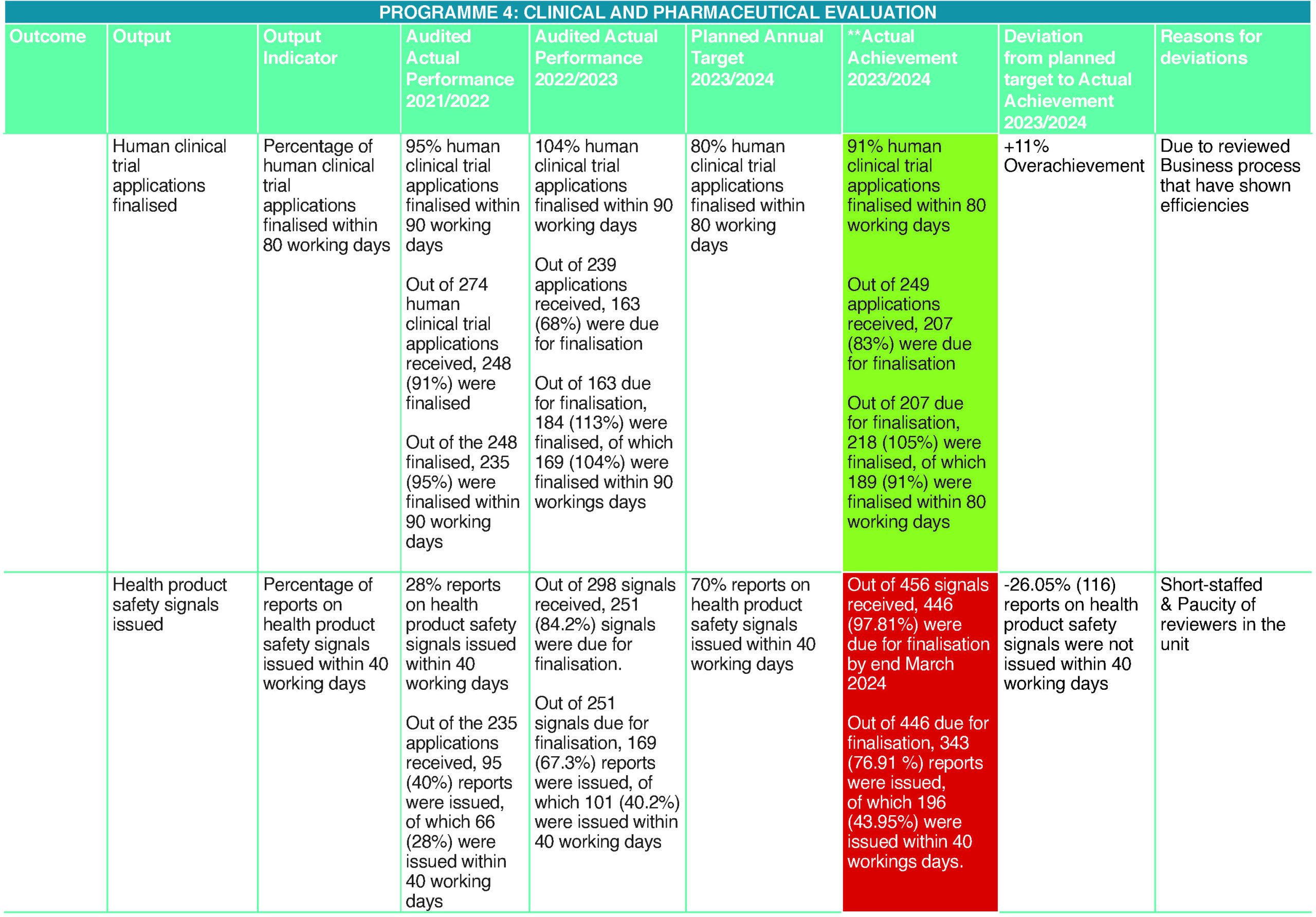
The Medicines Act (Act 101 of 1965, as amended) provides for the sale of unregistered medicines and other health products under certain circumstances. These include compassionate use for unmet medical needs. This occurs when a registered alternative is either unavailable or does not meet the identified medical needs of the patient. The Medicines Act, therefore, allows access to health products not registered in South Africa but available in other markets. This is a significant public health intervention to ensure prompt access to life-saving health products if these are not otherwise available to prevent disease complications. Ninety-six percent of applications for selling unregistered Category A (human) medicines were finalised within three working days. This is a result of consistent swift reviews of unregistered medicines enabled by an efficient in-house regulatory review team, including the senior manager, who continued to contribute to SAHPRA’s revenue-generation from the private sector, Section 21 applications as well as improved website information so that it is user-friendly and accessible.
Human Clinical Trials
To ensure Good Clinical Practices in clinical trials on humans, SAHPRA has regulatory oversight of human clinical trials conducted within South Africa. This oversight and monitoring ensure and facilitate the effective processing of clinical trial protocol applications to allow for approval of the conduct of clinical trials. Efficient approval of the conduct of clinical trials enables timely access to health research and development within an environment that guarantees the safety of clinical trial participants. Most trials received were in pulmonology, followed by oncology, and similar distributions in the following therapeutic areas: infectious diseases, including tuberculosis and Human Immunodeficiency Virus, and cardiology. The number of COVID-19-related clinical trials has tapered off significantly since April 2023.
Health Product Safety Signals
Medicines are not without risk. To this end, SAHPRA is mandated by the Medicines Act to monitor and evaluate the safety, efficacy, and quality of health products distributed and sold in South Africa. Such monitoring is comprehensive and responsive. Any signals of safety concerns and/or lack of clinical efficacy are evaluated promptly based on available evidence. Considerable effort is made to respond to safety signals despite inadequate resources. As a result of constrained resources, serious signals and those of high public health impact are prioritised and concluded. However, the set target of 70% in the 40 working days timeframe was not reached. Only 44% of the safety concerns received were finalised within 40 working days.
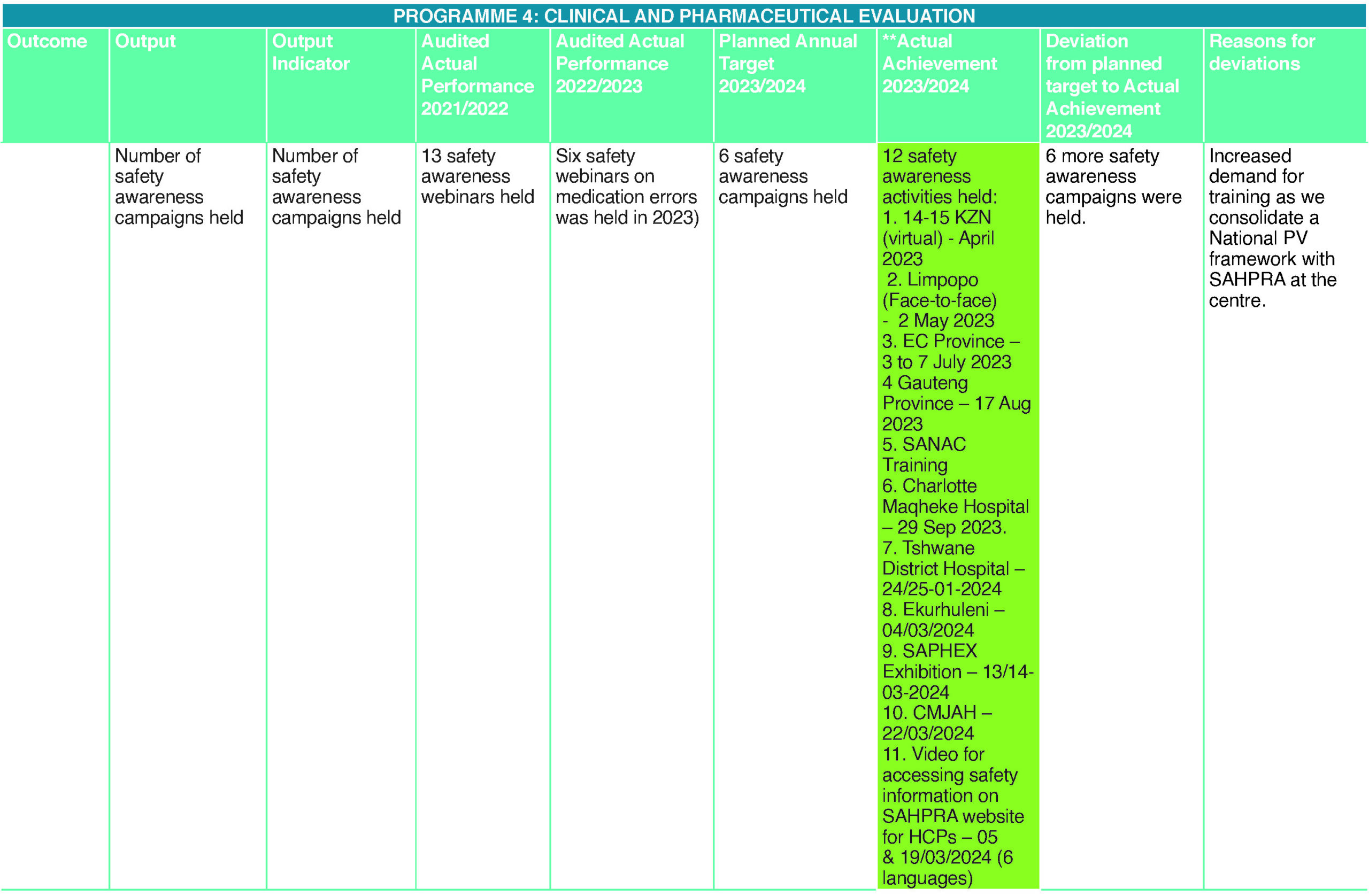
For the Authority to be aware of the safety issues, there is a need for healthcare professionals and consumers to report adverse events following the use of a medicine. SAHPRA has therefore developed, finalised and printed a pharmacovigilance training manual on three basic pharmacovigilance modules as it continues to intensify safety awareness and training healthcare professionals on these modules: the importance of pharmacovigilance, recognising ADRs in clinical practice and learning how to report.
The training manual covering the above modules was finalised and printed during the year under the review, which will be made available to HCPs trained in the above.
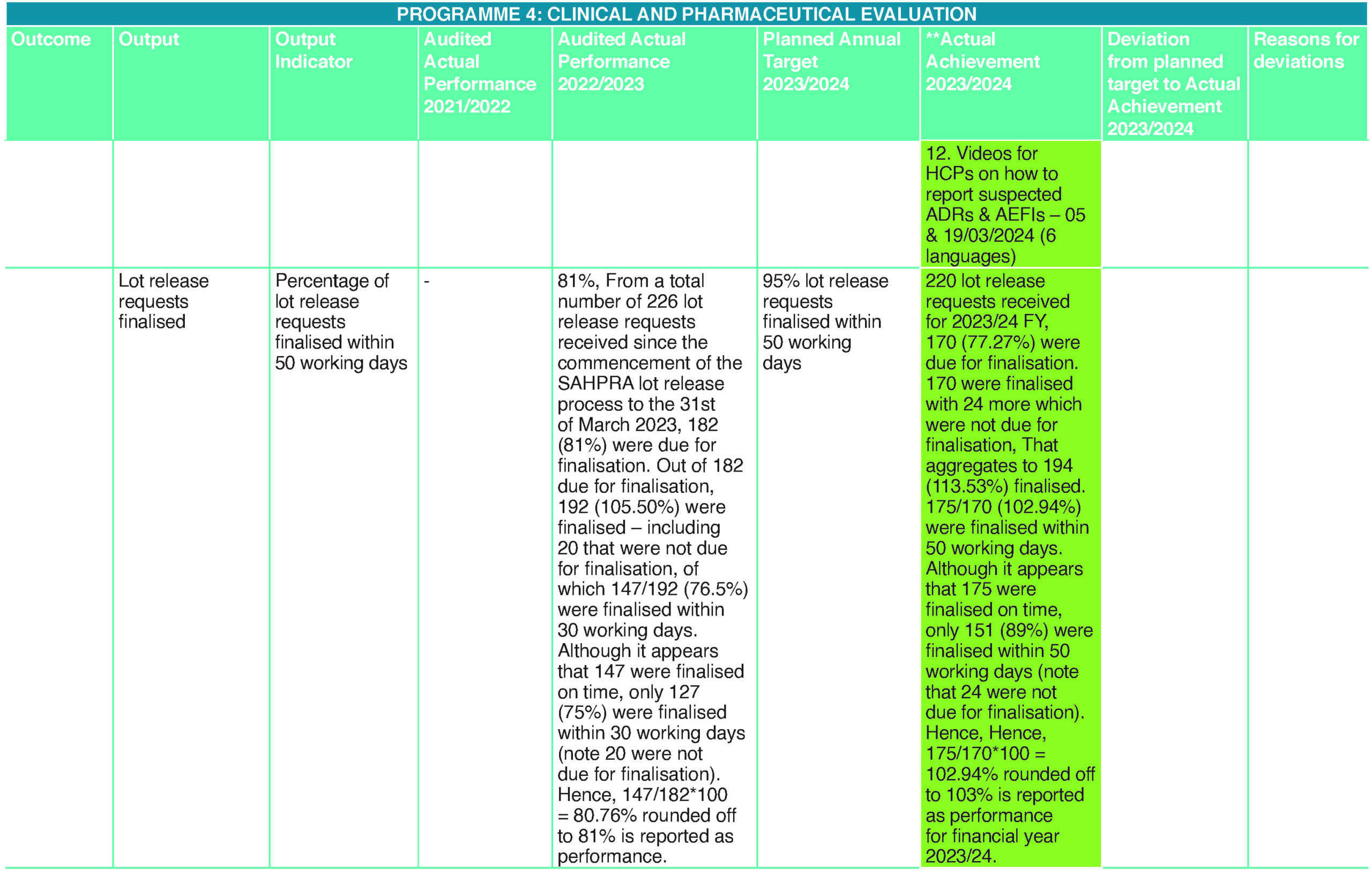
Linking performance with budgets:

Strategy to overcome areas of underperformance
The use of external regional reviewers for Quality and BE reviews to increase turnaround timelines and aid in reduced lag times in allocation with more reviewers’ presence. The implementation of the overtime to add value in more applications is to be finalised. Furthermore, capacitating the organisation with the right skills is significant in achieving the objectives of the Authority. The implementation of RIMS will continue together with the technology consultancy projects; in a nutshell, the digitation of the processes remains the priority of the organisation to ensure operational efficiencies.
Programme 5: Medical Devices and Radiation Control
Purpose: To develop and maintain regulations and guidelines on the regulatory oversight of medical devices, radionuclides, and listed electronic products.
Sub-programmes

Outcomes
Efficient and effective regulatory practices maintained (7)
Outcomes, Outputs, Output Indicators, Targets and Actual Achievements
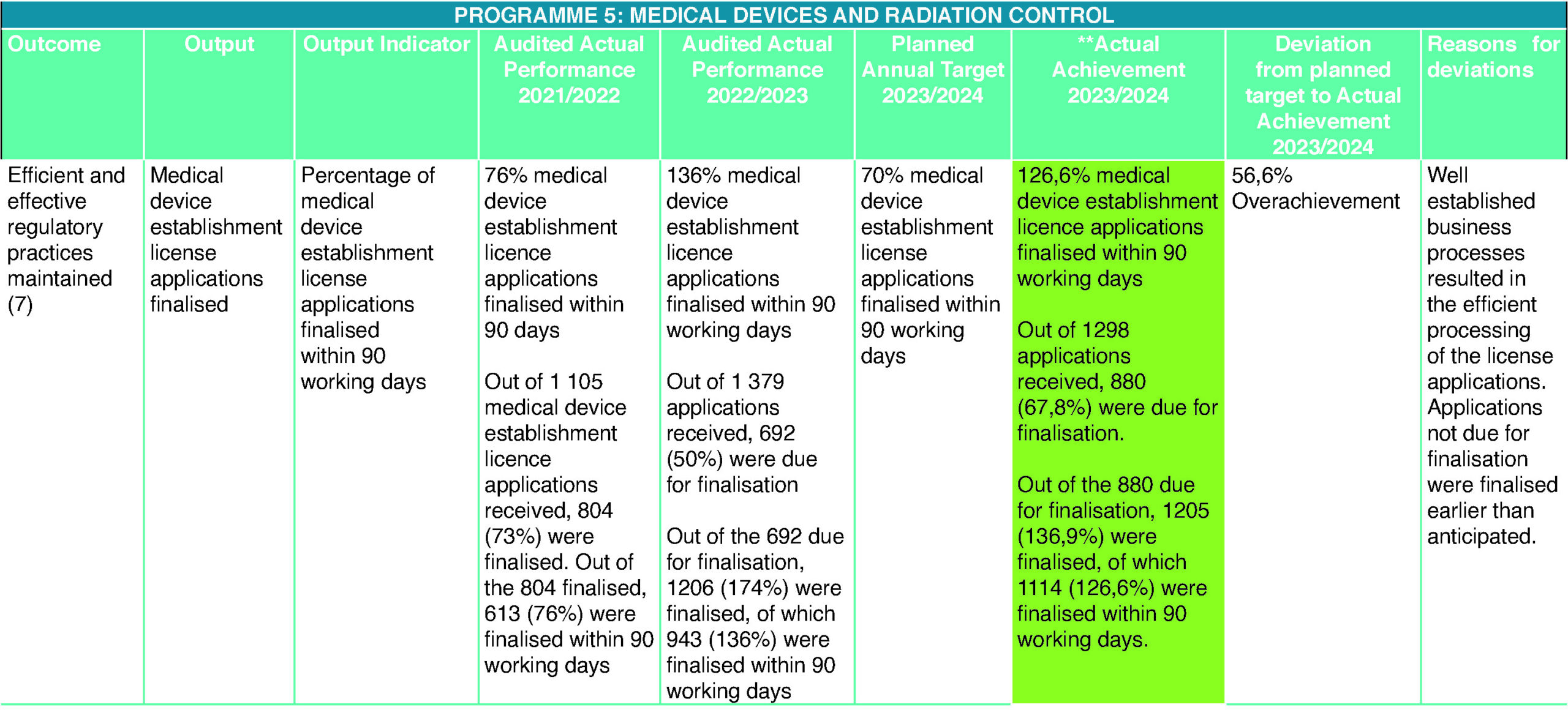
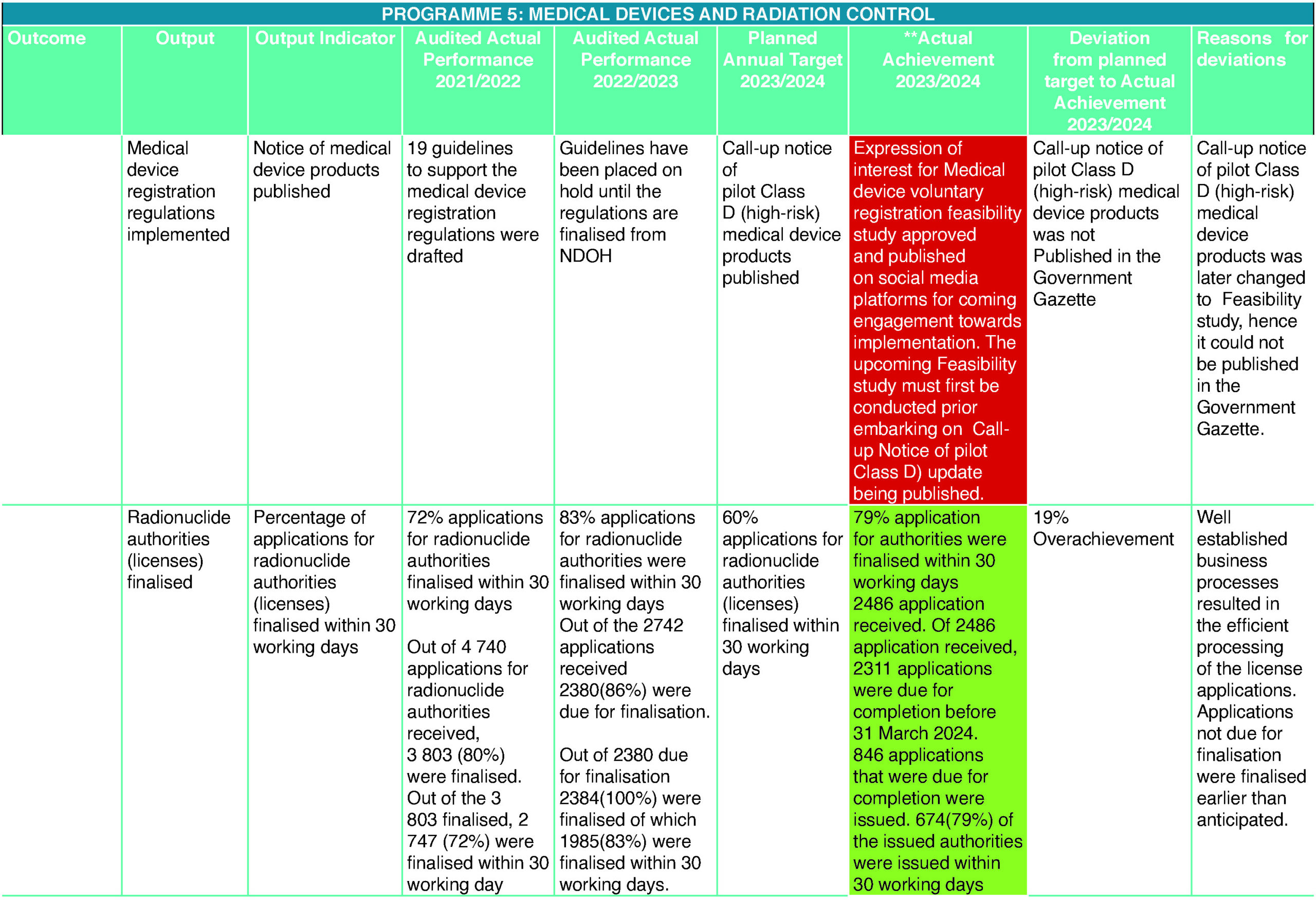
Medical Devices
The 2023/2024 financial year proved to be the most challenging year for South Africa in terms of allocation and utilisation of much-needed funds. SAHPRA, as a Schedule 3A public entity, faced similar challenges and had to cut down expenses, which affected a number of strategic and operational functions, such as training, the appointment and/or replacement of team members such as the Technical Officer: Licencing and Vigilance, while upholding the promise of increasing access to medical devices and IVDs that meet the requisite standards of safety, efficacy, and quality, to protect the health and well-being of South Africans, through the achievement of the APP targets. The results of the year under the review certainly highlighted all the efforts that the executive and management had committed during the 2022/2023 and 2023/2024 financial years to strengthening the business processes, yielded exceptional outcomes and saw the unit achieve over and above the expected results. These efforts continued to avert the creation of a new backlog and, in turn, guaranteed increased collection of licence fees.
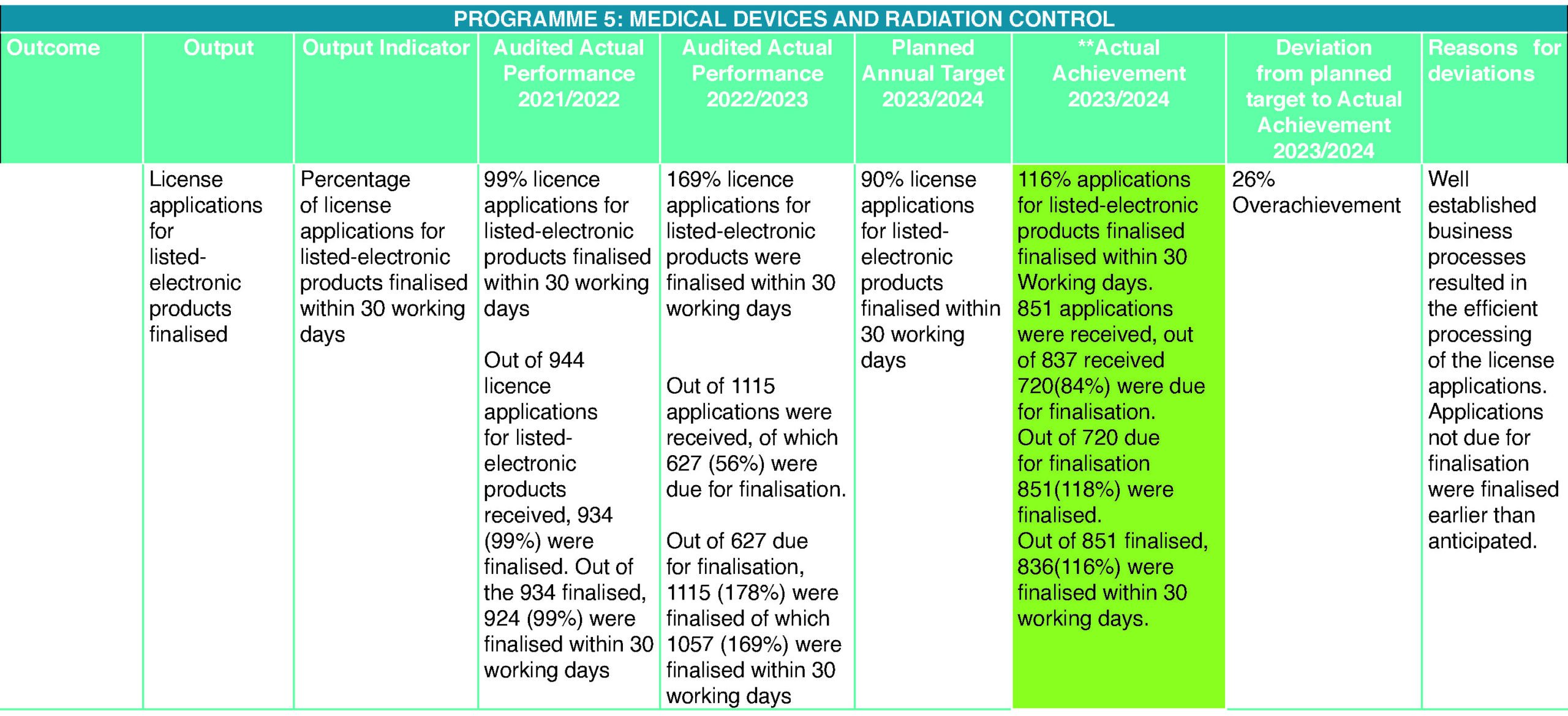
Radiation Control
The Radiation Control Subprogramme continues to improve its business process to achieve its annual performance plan targets. For the financial year 2023/2024, the team finalised 116% of applications of listed electrical products and 79% of applications of radionuclides authorities. The target of 90% of listed electronic product applications to be finalised within 30 working days was exceeded by 19%. The target of 60% of radionuclide applications for authorities was exceeded by 29%. The subprogramme continued to monitor, enforce, and improve compliance by completing applications of X-ray licences and performing inspections. For this financial year, the subprogramme completed over 500 X-ray applications and performed more than 300 inspections covering radiotherapy units, small dental units, diagnostic radiology units, nuclear medicine units, mines, and veterinary units.
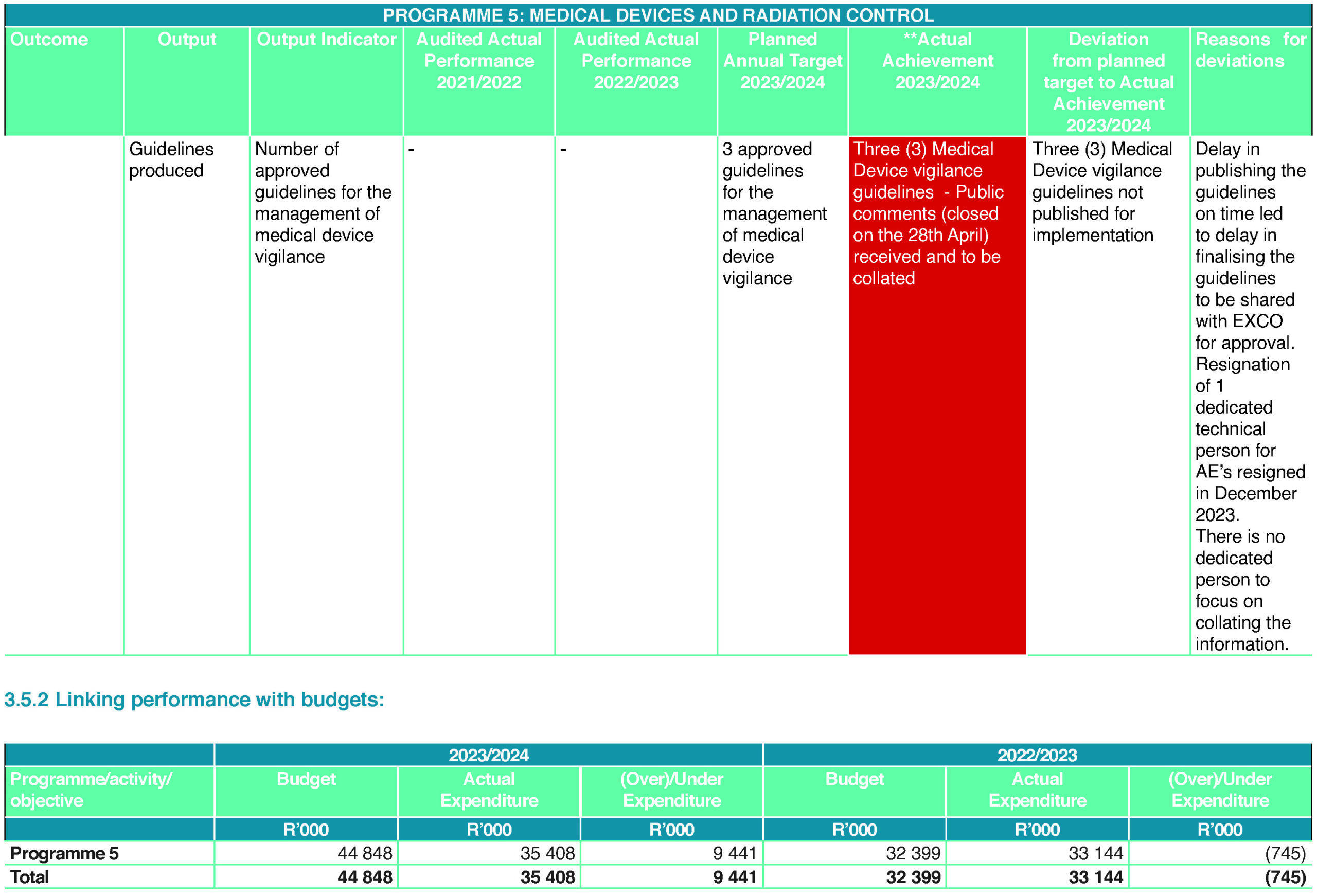
The continued improvement of the quality management systems (QMS) has helped the team finalise standard operating procedures (SOP) and guidelines. The subprogramme successfully completed 15 SOPs, migrated 23 guidelines to the new templates and published two new guidelines. The subprogramme participated in the QMS audit for the financial year 2023/2024 and received eight audit findings. The teams have completed and submitted all eight corrective actions requests forms and continue to work with the the QMS team for finalisation.
The subprogramme has also continued to improve awareness and compliance by engaging with various stakeholders. For the financial year 2023/2024, the team has engaged with these stakeholders: International Atomic Energy Agency (IAEA), South African National Accreditation System (SANAS), National Nuclear Regulator (NNR), Department of Mineral Resources and Energy (DMRE), National Regulator for Compulsory Specifications (NRCS), provincial departments of health in Limpopo, Mpumalanga, and the North West. The Medical Devices and Radiation Control team had stakeholder engagements with The Office of Radiation Safety and Oak Ridge National Laboratory where they held a workshop on the development of a guideline for security during the transportation of Category 2 and 3 sources.
While the subprogramme generally continued to improve in many areas, there has been a challenge of open vacancies where personnel have either resigned or retired. For this financial year, there are five open vacancies: Assistant Manager in Inspectorate based in Durban, Administration Screener for the Inspectorate subunit based in Durban, Inspectorate Assistant Manager based in Pretoria, Radiation Scientist for Non-ionising radiation and medical devices (NIRMED), Radiation Scientist for Radionuclides and Deputy Manager for Radionuclides and Radiation Control manager. The team has successfully managed to close the Inspectorate Assistance Manager post (Durban) and conducted interviews for the Inspectorate Administration Screener (Durban). For the Inspectorate Assistant Manager, the date for shortlisting is set. For NIRMED (Non-Ionising Radiation Devices) Radiation Scientist, the shortlisting is also set; for Radiation Scientist in Radionuclides, interviews have been contacted; for Deputy Manager in Radionuclides, interview dates has been set and Radiation Control Manager post will be re-advertised.
The drawback for the programme (both Radiation Control and Medical Devices) has been delays in closing of open positions and appointment of new people to support the operational activities.
Strategy to overcome areas of underperformance
Finalisation of the three vigilance guidelines, Licensing, Vigilance, and Compliance Manager to manage induction and operational training of the candidate. Furthermore, capacitating the organisation with the right skills is significant in achieving the objectives of the Authority. The implementation of the Regulatory Information Management Systems (RIMS) will continue together with the Technology Consultancy projects; in a nutshell, the digitation of the processes remains the priority of the organisation to ensure operational efficiencies.

